Cleft Palate
According to the National Center for Health Statistics, the rate of cleft palate alone in the United States in 2020 was 23.4 per 100,000 live births, while the rate of cleft lip with or without cleft palate was 49.5 per 100,000 live births. [1] The major morbidity of cleft palate is dysfunctional speech and communication impairment. A substantial number of children (approximately 20%) who undergo cleft palate repair develop a complex speech production disorder. As a result, speech dysfunction following palatoplasty is a significant problem. Appropriately, cleft surgeons have a concerted interest in postoperative outcomes, especially the occurrence and treatment of cleft-related speech dysfunction.
Cleft palate team
Current standards of cleft care include comprehensive multidisciplinary management by a qualified cleft palate team. The concept of an organized team evolved to address fragmentation of care by individual specialists. Once health professionals recognized the bewildering array of problems that an infant with facial cleft may develop, the concept of "team care" for children with such anomalies arose. The fundamental principle driving this approach involves sharing information and cultivating a global perspective of the patient's needs. The complexities of cleft lip and cleft palate anomalies make it necessary for a variety of clinicians to collaborate on planning and delivery of treatment. Members of disciplines as seemingly disparate as plastic surgery and speech pathology coordinate services to establish comprehensive management goals. [2]
Specialists from the 3 major areas of cleft care include medical and surgical (plastic surgery, pediatrics, nursing, social and psychological services), dental (pedodontics, prosthodontics, orthodontics, oral maxillofacial surgery), and speech and hearing (otolaryngology, audiology, speech therapy) specialists. This comprehensive team approach gives a child with a cleft the greatest opportunity for a pleasing appearance, healthy teeth, intact hearing, and intelligible speech. Additionally, the approach allows early identification of problems, should they develop, and limits the negative consequences of facial cleft deformities. The cleft palate team also addresses the complex social and psychological issues involved in the treatment of persons with these birth defects.
In the United States, cleft palate teams vary in location, scope of services, membership, and funding. More than 200 cleft palate teams exist in university-affiliated or freestanding tertiary medical centers, state public health facilities, and private-sector community locations. Following the model recently established in the United Kingdom, this figure will likely be reduced to just a handful of teams nationwide, each one of which manages a high volume of cleft-craniofacial anomalies. A team is within reach of every patient with a facial cleft. A code of standards has been formulated for teams around the United States, and a directory is published annually. Many states are in the process of legislating guidelines and standards and are adopting rules to minimize duplication of effort and promote general safety through documented management protocols.
How and when palatal clefting occurs
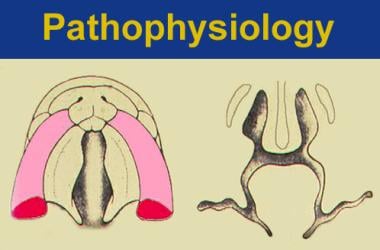
Facial tissues, including lip and palate, are produced from mesenchymal migration, penetration, and fusion of cranioneural crest cells. See the image below.
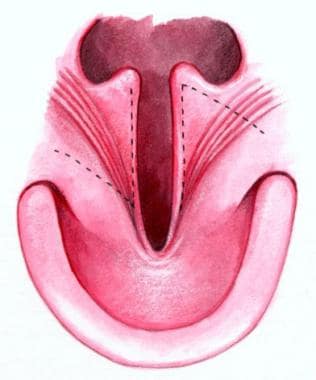
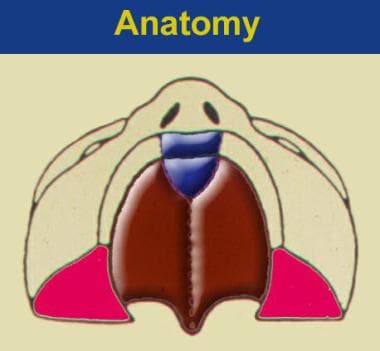
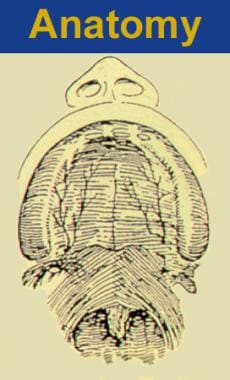 Two morphologically distinct parts comprise the secondary palate, soft tissue (posterior), and bony palatal components. Note the transverse orientation of the levator muscles. Also note the position of the incisive foramen separating the anterior structures, which are involved in the prepalatal (primary) palatal clefts, compared with the posterior structures, which are involved in the palatal (secondary) palatal clefts.
Two morphologically distinct parts comprise the secondary palate, soft tissue (posterior), and bony palatal components. Note the transverse orientation of the levator muscles. Also note the position of the incisive foramen separating the anterior structures, which are involved in the prepalatal (primary) palatal clefts, compared with the posterior structures, which are involved in the palatal (secondary) palatal clefts.
Each of the 3 main facial prominences (nose, lips, palate) is derived embryologically from bilateral converging facial processes. Formation of the primary palate begins at approximately 35 days of gestation. Complete lip development generally occurs by the sixth week of gestation, followed by palatal fusion. Three paired anatomic processes are involved with this phenomenon of migration and penetration: (1) the medial nasal process coalesces with the (2) maxillary process, followed by the coalescence of the (3) lateral nasal process with the medial nasal process (see the image below).
 Embryology processes of abnormal palatal development. Normally, the palatal shelves assume a vertical orientation and are directed downward. Free communication exists between the oral cavity and the nose at 7 weeks. Thus, the tongue is in direct contact with the inferior border of the nasal septum. The palatal shelves move horizontally as the process of fusion commences. Failure of fusion of the palatal shelves (medial extensions of the maxillary prominence), from any cause, may lead to cleft palate.
Embryology processes of abnormal palatal development. Normally, the palatal shelves assume a vertical orientation and are directed downward. Free communication exists between the oral cavity and the nose at 7 weeks. Thus, the tongue is in direct contact with the inferior border of the nasal septum. The palatal shelves move horizontally as the process of fusion commences. Failure of fusion of the palatal shelves (medial extensions of the maxillary prominence), from any cause, may lead to cleft palate.
Interference with the mechanism of mesodermal penetration may result in a cleft lip.
Development of the secondary palate begins after the primary palate has formed. This process is initiated when outgrowths (palatal shelves) push medially and bilaterally from the maxilla (see the image below).
 Development of the secondary palate. The process is initiated when outgrowths of the palatal shelves push medially and bilaterally from the maxilla.
Development of the secondary palate. The process is initiated when outgrowths of the palatal shelves push medially and bilaterally from the maxilla.
At first, the palatal shelves grow downward and adjacent to the tongue (see the image below).
Gradually, they elevate to a level above the tongue and assume a horizontal position (see the image below).
 Palatal shelves assume a level above the tongue and assume a horizontal position. Fusion of the 3 processes forms the normal secondary palate.
Palatal shelves assume a level above the tongue and assume a horizontal position. Fusion of the 3 processes forms the normal secondary palate.
Contact of these 3 processes and fusion of the tissues then occurs, resulting in the development of a normal secondary palate. If the palatal shelves fail to fuse, a cleft palate results. This mechanism may be the most common origin of cleft palate deformity.
Other possible reasons for palatal clefting involve abnormal growth of the palatal shelves, cell death (postfusion), and failure of mesenchymal consolidation and differentiation. Clefts of the secondary palate are usually the result of different morphogenic events when compared with cleft lip with or without cleft palate.
The following videos demonstrate animation of facial and palate development.
Causes of cleft palate
In 1963, Falconer established the multifactorial threshold theory as an attempt to explain the etiology of facial clefting. This theory proposes that clefting is directly related to hereditary and environmental factors involved in the development and growth process. Greater numbers of risk factors increase the probability of clefting. Falconer hypothesized that each case is unique, representing a combined liability or summation of risk factors. Once that composite liability exceeds a threshold, a cleft lip and/or palate will occur.
The precise meaning of the term threshold remains poorly understood; however, researchers know that various exogenous pharmacologic and environmental factors are responsible for clefting, including the administration of phenytoin, retinoids (from the vitamin A family), and steroids. Alcohol, hypoxia, and dietary deficiencies (ie, folic acid deficiency) also have been implicated. Polygenic clefting describes a number of different genes with additive effects that result in a genetic predisposition for clefting.
Molecular biology may have implications for future diagnosis and treatment of cleft lip and cleft palate. Single and multiple gene abnormalities are providing a framework for testing the association between DNA sequence variations in multiple candidate genes. For example, alleles of the gene encoding transforming growth factor (TGF) have been associated with cleft lip and palate. High levels of TGF have been detected in the medial edges of the palatal shelves at the time fusion in mice. Additionally, a high prevalence of cleft palate is reported in mice that are deficient in receptor TGF. These data provide biological support for the role of this growth factor in normal facial morphogenesis. [3]
Different inherent patterns for cleft palate by race, sex, and syndrome
The true incidence of cleft lip and palate is controversial. Current genetic research does not segregate cases by syndromic involvement. Despite this, awareness of the categories (clefting with or without syndromic involvement) is important to provide appropriate genetic counseling to the affected individual and his or her family. The general prevalence of cleft lip with or without cleft palate is believed to be 1 case per 700 live births. The prevalence of nonsyndromic cleft palate alone is believed to be lower (1 case per 2000 live births).
The distribution of clefting is influenced by the sex of the individual. The occurrence of nonsyndromic cleft lip with or without cleft palate is higher among males, while nonsyndromic cleft palate is more common among females. The rate of occurrence in one monozygotic twin is as high as 50%. The prevalence of nonsyndromic cleft palate differs little across racial lines; however, distribution of cleft lip with or without cleft palate varies widely with ethnicity. Analysis of data indicates that Native Americans have the highest prevalence of this type of facial cleft, while blacks have the lowest prevalence. Nonsyndromic cleft palate occurs as follows:
-
1 case per 1500-3000 whites
-
1 case per 200-5000 blacks
-
1 case per 1600-4000 Asians
-
1 case per 1700 Native Americans
Estimate of cleft lip with or without cleft palate is as follows:
-
1 case per 775-1000 whites
-
1 case per 1300-5000 blacks
-
1 case per 400-850 Asians
-
1 case per 230-1000 Native Americans
Twice as many males as females are affected by this condition. In contrast, nonsyndromic cleft palate occurs in an equal male-to-female distribution.
Cleft Palate Appearance
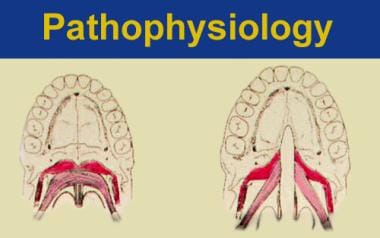
Cleft palate that accompanies a cleft lip usually appears long and narrow, while solitary clefts of the secondary palate appear as a U- or V-shaped cleft. The spectrum of morphology of a cleft palate ranges from simple notching to entire clefts through the alveolus (see the image below).
 Spectrum of cleft palate morphologies (from upper left panning to right). A. Normal development. B. Unilateral clefting of the lip. C. Unilateral clefting of the lip and primary palate. D. Bilateral clefting of the lips and primary palate including the incisive canal. E. Secondary palate cleft including the incisive canal. F. Unilateral clefting of lip, primary palate, incisive canal, and secondary palate.
Spectrum of cleft palate morphologies (from upper left panning to right). A. Normal development. B. Unilateral clefting of the lip. C. Unilateral clefting of the lip and primary palate. D. Bilateral clefting of the lips and primary palate including the incisive canal. E. Secondary palate cleft including the incisive canal. F. Unilateral clefting of lip, primary palate, incisive canal, and secondary palate.
The most common type of cleft palate is a bifid uvula, a simple cleft that splits the uvula into 2 sections. This type of cleft is functionally inconsequential and is present in 2% of the American population; however, a bifid uvula may signal the presence of a submucous cleft lip, which may be the source of pathologically incurred nasal resonance in a small percentage of people affected by it.
Submucous cleft palate
Calnan describes 3 typical physical findings associated with submucous cleft palate. [4] As mentioned above, a bifid uvula may herald the presence of a submucous cleft palate. The other 2 anatomic features include a very thin membranous central portion of the soft palate (zona pellucida) and diagonal ridges located lateral to it. A palpable notch located at the junction of the hard and soft palate, the posterior nasal spine, may corroborate the latter finding.
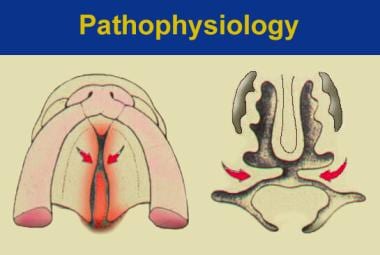
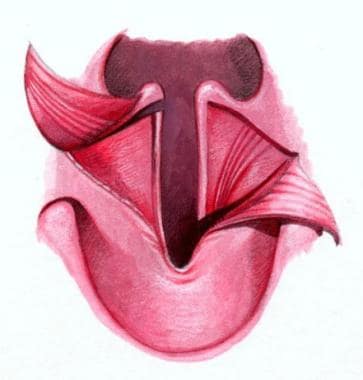
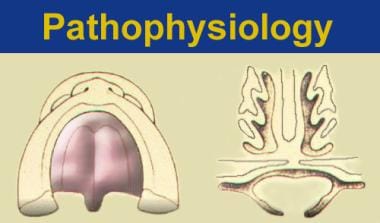
The structural presentation of submucous cleft palate indicates that the levator muscles have been shifted from their normal transverse orientation to a longitudinal position (see the image below).
 The left illustration is of normal soft palatal anatomy. Notice the transverse orientation of the levator muscles across the posterior portion of the soft palate. The right illustration shows cleft anatomy, in which the levator muscles are orientated more longitudinally and insert on the posterior edge of the palatal bone and along the bony cleft edges.
The left illustration is of normal soft palatal anatomy. Notice the transverse orientation of the levator muscles across the posterior portion of the soft palate. The right illustration shows cleft anatomy, in which the levator muscles are orientated more longitudinally and insert on the posterior edge of the palatal bone and along the bony cleft edges.
The muscles, presumably important for normal speech, insert aberrantly on the bony free edge of the hard palate instead of forming a complete muscular sling in the soft palate.
Although all 3 components need not be present to establish the diagnosis, Kono et al report an increased prevalence of this condition in patients with cleft lip alone. [5] Many submucous cleft palates remain undiscovered until early adulthood, because the patient's speech usually is unaffected. Surgical intervention was not indicated for 44% of 130 patients with submucous cleft palate evaluated by McWilliams. [6] Other studies in the literature confirm these findings.
Conversely, hypernasality, nasal emission, and facial grimacing are symptoms that may occur in 10% of patients with submucous cleft palate. Adenoidectomy in these patients should be undertaken cautiously. Surgeons are strongly advised to observe patients closely for the development of speech production disorders. If these patients require surgical management, the clinician should wait until speech patterns are established and the patient has undergone a complete speech/velopharyngeal evaluation by a qualified speech physiologist. Counsel parents that if an adenoidectomy is necessary to facilitate technical execution of the procedure, it may actually worsen speech symptoms or unmask previously undetectable clinical manifestations.
Difference between cleft types
The variations in structure among cleft types are related to the different mechanisms involved in the clefting process. With a complete unilateral cleft lip and palate, the vomer is always attached to the noncleft side of the hard palate. This occurs because the palatal shelf on the cleft side of the tongue usually reaches its horizontal position prior to the noncleft side.
The cleft palate observed in the Pierre Robin malformation sequence is generally a U-shaped cleft of the secondary palate. This morphology may result from a delay in the descent of the tongue from its position between the palatal shelves or from a delay in the cephalic flexion of the embryo. The heterogeneity among clefts indicates an inherent variability of embryonic dysmorphogenesis.
Isolated cleft palate
In common parlance, the term isolated cleft palate is a cleft palate without a cleft lip. Geneticists oppose the use of the term isolated cleft palate. Dysmorphologists use the term to indicate that clefting exists "without any additional congenital anomalies." Some authors believe that virtually all patients with posterior cleft palate have additional malformation syndromes, even if they have not yet been diagnosed.
Cleft palate classification
The following is the 4 categories for organizing cleft palate cases based on the variations of their supposed inheritance patterns:
-
Nonsyndromic cleft lip with or without cleft palate
-
Nonsyndromic cleft palate
-
Syndromic cleft lip with or without cleft palate
-
Syndromic cleft palate
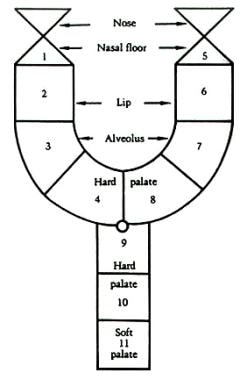
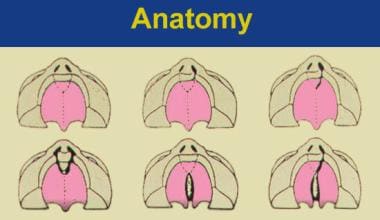
Kernohan has provided a pictorial description, the striped Y classification for cleft lip and palate. This system was modified by Millard to include descriptions of the nasal tip and nasal floor (see the image below). [7]
How Does Cleft Palate Affect Hearing and Speech?
Middle ear disease is virtually ubiquitous among individuals affected by cleft palate. The hypothesis for the high prevalence of serous otitis media relates to the putative abnormal insertion of tensor and levator veli palatini muscles. These muscles insert variably on the eustachian tube. Both are probably responsible for competence of the tube in preventing reflux from the nasopharynx into the eustachian tube, as well as for opening the tube to equalize pressure in the middle ear.
Chronic ear infections, related to eustachian tube dysfunction, [8] may account for hearing loss in some patients with cleft palate. Whether cleft palate repair is effective in diminishing the occurrence of otitis media in these patients is unknown. Click here to complete a Medscape CME activity on chronic otitis media.
Cleft palate affects speech
A Korean study found that children aged 9-18 months with cleft palate demonstrated lower proportions of advanced vocalization levels, as represented by canonical and complex syllable structures, than did children in the same age range without cleft palate. [9]
Phonations requiring velopharyngeal closure result in an isometric contraction of the levator muscles. This finding has been observed in overt clefts and in classic submucous cleft palate. Braithwaite and Maurice, in the mid-1960s, emphasized the anomalous anatomy of the levator veli palatini muscles present in the cleft palate. [10] They recognized that these muscles were actually inserted into the edges of the bony cleft instead of simply joining in the midline, as observed in normal palatal anatomy.
Kriens emphasized the abnormal orientation of the levator palatini muscles and the need to detach them from their abnormal insertion and reorient them in a transverse direction. The velopharyngeal sphincter is essential for production of nonnasal sounds. When closed, the ability to allow airflow into the nasal chamber is diminished. Thus, the primary purpose of cleft palate repair is to create a velopharyngeal valve responsible for directing sound energy and airflow from the pharynx into the oral cavity for oral sounds and into the nasal cavity for nasal sounds.
Speech impairments specific to velopharyngeal dysfunction (VPD) include the inability to produce sibilant consonants, including the sounds s, z, sh, zh, and ch; j or soft g; and the intraoral pressure consonants p, b, t, d, k, and hard g. Patients attempt to compensate for their inability to close the velopharynx with maladaptive articulation patterns. The resulting abnormal resonance (hypernasality) and poor articulation (mechanical inability to produce appropriate sounds) can cause unintelligible speech. Speech therapy to optimize velopharyngeal function is usually necessary in children aged 4-10 years. Improved resonance can help articulation and other important components of speech.
Hypernasality is a resonance disorder that occurs when there is abnormal coupling (sharing of acoustic energy) of the oral and nasal cavities during speech. Specifically, it is abnormal nasal resonance during the production of non-nasal oral sounds.
Nasal air emission results from an attempt to build up intraoral air pressure for the production of consonants in the presence of a leak in the system, either from a fistula or from the velopharyngeal valve. Some of the airflow is released through the nose, causing a disruption in the aerodynamic process of speech. Nasal emission is detectable on pressure sensitive phonemes, plosive fricatives, and affricates, not on vowels. It can occur with normal resonance. Whether the emission is audible or not depends on the size of the opening (the bigger the opening, the less resistance to the flow).
Differentiating between speech function and valvular function is very important. One should not operate on a potentially competent valve, nor waste resources and time on speech therapy when the valve is incompetent. The 2 important clinical criteria for judgment of complete closure are (1) elimination of nasal escape and (2) hypernasality. The surgeon is responsible for providing a velopharyngeal mechanism that is capable of quick, efficient, and complete closure of the valve for speech. The speech pathologist is responsible for helping the patient achieve speech and language success, including articulation and other facets of verbal communication.
Cleft palate repair affects normal facial growth and development
The effect of palatal surgery on maxillofacial growth is observed most clearly in persons aged 10-20 years. Although the patient may have a normal appearance early in life, he or she may begin to develop a definite flattening of the mid face and dentoskeletal malocclusion, particularly during adolescence.
Speech impairments affect a patient's social status
The importance of socially acceptable speech for equal societal opportunity was evaluated and emphasized in a study conducted by Neiman and Duncan, which compared the social and vocational acceptability of individuals with cleft lip (both with and without VPD) to individuals with no cleft anomalies. [11] In the study, potential employers rated the individuals. Results of the study indicated that VPD produces a strong negative impact on the selection of employees for prestigious jobs, even more than the presence of a facial disfigurement.
What Are the Key Anatomic Features of the Palate?
By convention, the primary palate consists of tissues anterior (ventral) to the incisive foramen (see the image below).
 Two morphologically distinct parts comprise the secondary palate, soft tissue (posterior), and bony palatal components. Note the transverse orientation of the levator muscles. Also note the position of the incisive foramen separating the anterior structures, which are involved in the prepalatal (primary) palatal clefts, compared with the posterior structures, which are involved in the palatal (secondary) palatal clefts.
Two morphologically distinct parts comprise the secondary palate, soft tissue (posterior), and bony palatal components. Note the transverse orientation of the levator muscles. Also note the position of the incisive foramen separating the anterior structures, which are involved in the prepalatal (primary) palatal clefts, compared with the posterior structures, which are involved in the palatal (secondary) palatal clefts.
The primary palate includes the nose, lips, prolabium (center of the upper lip), and premaxilla (anterior maxilla including 4 incisors), while the secondary palate consists of most of the hard palate and the entire soft palate.
The 6 muscles of the palate are the levator veli palatini, superior constrictor pharyngeus, palatoglossus, uvula, palatopharyngeus, and tensor veli palatini.
Of these, the levator palatini muscles probably are most important for speech. They move upward on both sides of the base of the skull from the vertex of the petrous portion of the temporal bone. These muscles elevate the soft palate and, along with the superior constrictor muscles, are credited for medial movement of the lateral pharyngeal wall. The superior constrictor of the pharynx and the uvular muscle assist in velopharyngeal closure by increasing the mass of the soft palate on the nasal surface as it contracts in the posterior direction.
Function of the palate
The hard portion of the secondary palate serves as a static partition between the oral and nasal chambers for feeding, while the soft palate (containing mostly musculature) serves as a dynamic barrier between the mouth and nose, intermittently valving for speech and airway. Velopharyngeal valving facilitates swallowing and appropriate speech production of essentially all vowels and consonants in the English language, with the exception of m, n, and ng.
When Should a Cleft Palate Be Repaired?
The timing of palatal repair has incurred lively debate. As with all operations, there is a finite incidence of surgical failure following palatoplasty. Reasons for failure are complex because they include factors such as deafness or associated malformation syndromes, which are responsible for a certain number of poor results. Additionally, most physiologic functions are best learned at an early age. Neuromuscular control, synchronization, and integration into the neurologic system should occur at the optimal time; otherwise, ineffective coordination and abnormal compensatory habits may result. For example, a child deprived of light perception early in life as a result of hemangiomas, congenital cataracts, or other conditions that alter light perception may develop amblyopic blindness, loss of visual acuity, or nonconjugate vision, even though he or she may have no anatomic abnormalities in the obstructive eye.
Speech-dependent functions are also known to be influenced by age. A child who is deprived of hearing during the first few years of life does not develop normal speech, even if hearing is later corrected. The longer the period of sound deprivation, the greater the speech abnormality. Similarly, the possibility exists that palatal speech functions have an optimal time for normal development; abnormal patterns become linguistically embedded and perhaps uncorrectable if the palate is repaired beyond that time.
The issue of how early a palatoplasty should be performed remains unresolved. Addressing this issue will likely involve strict adherence to the scientific method and multicenter prospective studies. Future investigations in this area are needed to examine the effects of surgery in discrete time frames. Information is needed that documents the stages to which both articulation and language can develop presurgically without compromising the postsurgical speech result. In addition, information is needed on the distribution of patients within each stage who retain stigmata of VPD. To date, no studies have conclusively shown that palate repair before age 6 months improves speech outcomes.
The decision regarding optimal timing of palatal surgery is important, and it probably should not be dominated by a rigidly defined chronological standard. If evidence ever shows that children are better served if they undergo the operation at a certain age, that age should be expressed as a developmental age, not a chronological age. The important factor remains the child's level of speech and language performance at the time of surgery, not his or her age.
The dilemma regarding timing of palatoplasty involves how clinicians weigh the morbidity of impaired speech intelligibility (when palatoplasty is delayed) against improved facial growth. The main questions at the center of the debate include the following:
-
Can proper speech production and satisfactory maxillofacial growth be achieved synchronously after palatal repair?
-
What is the optimal age for surgical intervention for a patient with cleft palate, so that neither speech dysfunction nor growth disturbance occurs?
To provide optimal speech results, most health care providers accept performing palatal repair before the child develops meaningful speech. Palate closure should occur before age 18 months. Historically, several different timing protocols have been advocated, as follows:
-
Early soft palate repair at age 6 months, followed by delayed repair of hard palate at age 6 years
-
Delayed complete repair of the palate at age 12-24 months
-
Complete repair of palate when the patient is younger than 12 months
A study by Gamble et al found that in medically fit infants in adequately resourced settings, the rate of velopharyngeal insufficiency was lower in those who underwent surgery for isolated cleft palate at age 6 months than in those in whom repair was performed at age 12 months. At 5-year follow-up, the rates of velopharyngeal insufficiency in the 6-month and 12-month surgery groups were 8.9% and 15.0%, respectively. [12]
A study by Paine et al reported a 2.8% complication rate at 30 days postsurgery for patients aged 3 years or under who underwent primary cleft palate repair. Risk factors for complications were found to include oxygen support, nutritional support, structural pulmonary/airway abnormality, esophageal/gastric/intestinal disease, cognitive impairment, concurrent surgery, concurrent laryngoscopy, and an American Society of Anesthesiologists physical status classification of 3. The study, which employed the American College of Surgeons Pediatric National Surgical Quality Improvement Program database, involved 751 patients, including 21 with complications. [13]
A retrospective cohort study by Smyth and Wu found that of 271 nonsyndromic infants who underwent a cleft palate repair involving levator veli palatini muscle repositioning with or without lateral palatal release, fistulae occurred in 28 patients (10.3%) followed up at age 5 years. The investigators also found that the existence of fistulae led to a three-fold increase in velopharyngeal insufficiency, with the rate rising from 18% to 54%. The velopharyngeal insufficiency rate reached 71% when fistulae complicated bilateral cleft lip and palate repair. [14]
Rationale for delayed palatoplasty
In 1944, Schweckendiek hypothesized that repairing the soft palate at age 6 months and the hard palate at age 12-16 years would allow proper speech to develop while at the same time preventing maxillary growth disturbance. Theoretically, this protocol delays mucoperiosteal stripping and thus attenuates growth disturbances. The long-term results of this approach were published in 1978 by Schweckendiek's son. [15] The protocol proved to be salutary for facial growth, but only 28% of patients achieved normal speech. This study, termed the Marburgproject, evaluated 43 randomly selected patients from the Schweckendiek series. Cephalometric (dentoskeletal) data confirmed that 88% of patients experienced normal facial growth, but VPD was present at a rate of more than 1 in 2 patients (54%) evaluated.
Several studies have concluded that delaying surgery to complete hard palate repair in children older than 18 months results in unsatisfactory speech outcomes, while earlier repair produces no significant difference in facial growth compared with delayed repair. [16] Optimal facial growth results are achieved when patients undergo palate repair at age 14 years; following closely are those who undergo repair at age 1 year. In a study of 57 patients who underwent entire cleft palate repair by age 1 year, Enemark et al found some growth impairment but obtained normal and socially acceptable profiles in 50 patients. [17]
Rationale for early palatoplasty
Many authors argue that early repair obtains the best results for speech. Early operation allows the infant to experiment with different sounds (ie, babbling). A study by Dorf and Curtin supports surgical intervention in patients younger than 6 months. [18] Their research compared the speech performance of patients who underwent palate repair before and after 1 year. They concluded that the best results for speech performance were obtained when children underwent early palate repair. Only 10% of those studied developed compensatory articulation, as voiced by Trost. [19] Conversely, a disproportionate 86% of those patients observed who underwent palatoplasty later developed these maladaptive articulation patterns. Importantly, note that the research design of this report has been heavily criticized and many clinicians do not trust its results.
A similar study on the timing of palatoplasty was performed by Peterson-Falzone, who reviewed the records of 3 cleft palate clinics. [20] Analysis of results did not reach statistical significance, yet she found a higher incidence of unintelligible speech among those who underwent early management. Dalston concluded that no speech-related reason exists for surgeons who perform repair on children aged 12-14 months to change their timing protocol. [21]
When to repair clefts observed in the Pierre Robin sequence
Patients with Pierre Robin sequence (microretrognathia) often present with histories of perinatal respiratory and feeding difficulties. When management of the airway is no longer necessary and substantial craniofacial development is obvious, repair the palate. Lehman et al found that complications after palate repair were most common among patients who had difficulties during the neonatal period. [22] Complete closure of the palate should occur at or near age 12 months to achieve optimal results and minimize the possibility of maxillary growth disturbances.
Treat patients with Pierre Robin sequence no differently than other children with cleft palate, except approach the operation with a heightened sense of awareness of looming respiratory problems and counsel parents appropriately. A wise plan may be to admit these children to the intensive care unit for overnight airway monitoring and management.
A study by van Lieshout et al found that 30% of children with Pierre Robin sequence who underwent cleft palate repair developed respiratory distress, although the problem was temporary in all of them. In the study, palatoplasty was performed on 30 children with Pierre Robin sequence and on 45 without it, with respiratory distress arising postoperatively in nine of the Pierre Robin sequence patients (but resolving within a few days), while occurring in none of the other group. [23]
Techniques used to repair palate
Three distinct surgical methods are used for closure of the cleft mucoperiosteum. The approach probably is less important than the surgeon's cumulative operative experience. Currently, data in the surgical literature are insufficient to compare these approaches.
-
Bernard Von Langenbeck is credited with pioneering the first bipedicle mucoperiosteal flaps for palate closure. Almost every repair undertaken today is influenced by this important contribution. His approach involves elevating bipedicle mucoperiosteal flaps.
-
More recently, within the last 100 years, Veau focused on the importance of lengthening the palate. The Veau-Wardill-Kilner "pushback" theory achieves this length by increasing the movement of the posterior tissues through elevating bilateral peninsular flaps, dissecting the greater palatine neurovascular bundles, and occasionally fracturing the hamulus in an effort to achieve the greatest length possible. Unlike the classic Von Langenbeck repair, the mucoperiosteum is detached anteriorly at the premaxilla. Subsequently, 4 converging suture lines are created.
-
The third approach, the Furlow double-opposing Z-plasty, depends, alternatively, on the inward mobilization of the mucoperiosteum. Essentially, it is a soft palate operation because no relaxing incisions are made. Anecdotally, increasing numbers of surgeons seem to be using the double-opposing Z-plasty exclusively.
A literature review by Stein et al reported that the risk of fistula formation is lower with the Furlow technique than with the Von Langenbeck and Veau-Wardill-Kilner procedures, while the likelihood of velopharyngeal insufficiency is less with the Furlow operation than with Bardach repair (discussed below). [24]
D'Antonio et al, based on preoperative and postoperative assessments of velopharyngeal function, have also provided evidence to support the use of the Furlow procedure. [25] Their report prospectively documented anatomic changes associated with this procedure. Radiographic dimensions of the velopharynx and aerodynamic measures of velopharyngeal function were described in a group of 12 patients before and after Furlow Z-plasty for the treatment of velopharyngeal insufficiency. Cephalometric radiographs were used to measure velar length, velar thickness, and pharyngeal depth. Mean nasal airflow during pressure consonants (Vn) was calculated from pressure-flow studies, and patients were categorized as having complete closure (< 10 mL/s Vn) or incomplete closure (>10 mL/s Vn).
After Z-plasty, a significant increase in velar length (P = .002) and velar thickness (P = .001) occurred. After surgery, patients with complete velopharyngeal closure had significantly greater velar length compared with the incomplete closure group (P = .05), with nearly twice the increase in length. Similarly, following surgery, the complete closure group had significantly greater thickness than the incomplete closure group (P = .01), with a greater postoperative increase in velar thickness (P = .005). Finally, a significant negative correlation was shown between percent increase in length and percent increase in thickness for patients in the complete closure group (r = -0.91, P = .03).
Findings demonstrate that following Furlow Z-plasty, patients with cleft palate and velopharyngeal insufficiency obtained significant increases in velar length and thickness. Greater velar length and greater velar thickness both were associated with complete velopharyngeal closure. Patients in the complete closure group tended to demonstrate large percent gains in either length or thickness or moderate gains in both.
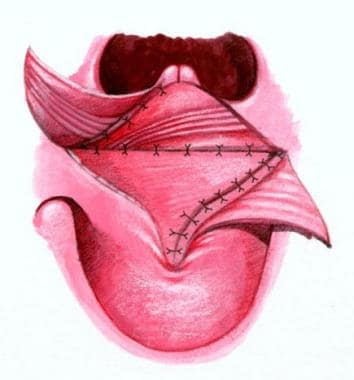
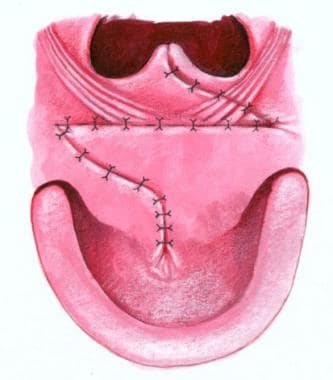
Furlow palatoplasty technique
See the images below.
The incision lines are plotted with indelible ink. The soft palate is divided from the uvula tip to the junction of the hard and soft palates, and the incision is extended approximately 1 cm toward the incisive foramen. The lateral lengths of the oral Z-plasties are drawn to end over the hamuli, which can be palpated with a finger. The lateral limb of the posteriorly based flap is designed to run from the junction of the hard and soft palates. The anteriorly based flap is designed to run from the uvula to the hamulus.
The cleft margin is incised along the visible junction line between the oral and nasal mucosae in the soft palate and exactly on the cleft margin in the hard palate. Mucoperiosteal flaps are elevated through the cleft with a Blair elevator. Lateral relaxing incisions are not made.
On the left, the lateral limb incision is made, and the tip of the flap is elevated with a knife. The palatal muscle is detached from the margin of the hard palate. Along the relatively fibrous cleft margin, the muscle is separated from the nasal mucosa with curved scissors and a No. 15 blade knife as needed. When the dissection reaches a point just medial to the hamulus, the palatal aponeurosis is completely divided. A Freer elevator is then used to separate the palatal muscle from the superior constrictor fibers just lateral to it, freeing the flap for rotation.
Complete muscle separation from the nasal mucosa exposes the mucosa for the lateral limb incision of the nasal anteriorly based Z-plasty flap. The incision extends from anterior to the uvula to the tip of the eustachian tube orifice, which is located by dropping the tip of the Freer elevator into it. The right side of the cleft is incised, and the mucoperiosteal flap is also elevated. Care is taken to elevate the mucoperiosteum around the anterior end of the cleft to separate the oral and nasal mucosae in order to avoid a fistula.
On the right side, a lateral limb incision for the anteriorly based oral Z-plasty flap is carried through the mucosa, and the flap is elevated from the underlying muscle. The mucosa is fairly thin along the margin of the cleft, but the dissection is deepened to include minor salivary glands more laterally, making the flap somewhat thicker. The flap base is elevated by joining the subcutaneous plane of the flap dissection with the mucoperiosteal plane along the posterior margin of the hard palate, and mobilization is performed with the Freer and Blair elevators around the greater palatine vessels.
The nasal right-sided posteriorly based flap is then elevated. The palatal muscles are detached from the hard palate. The eustachian tube orifice is identified. The nasal mucosal incision is made, leaving a free edge in the nasal mucosa. The palatal aponeurosis is divided, exposing the flap's palatal muscle for separation from the superior constrictor fibers lateral to it.
Closure of the nasal side is then begun with 4-0 Vicryl, suturing the uvular tags with horizontal mattress sutures to minimize notching of the uvula. The tip of the posterior nasal flap is then inset into the lateral end of the lateral limb on the left side of the eustachian lip, bringing the right palatal muscle across the cleft. The mucosal margins between the uvula and the tip of the flap are then closed. The nasal mucosa flap is then brought across the cleft and inset into the right side in a similar fashion.
Oral closure with 4-0 Vicryl is then begun by transposing the posteriorly based flap that overlaps the palatal muscles to form the palatal muscle flank. The anteriorly based flap on the right side is then brought across the cleft. The flap is somewhat difficult to mobilize adequately, and a small back-cut from its lateral end medially around the posterior margin of the alveolus improves mobility and facilitates dissection around the greater palatine vessels. Horizontal mattress sutures are then used to evert the stiff mucoperiosteum on the hard palate for closure. The closure is carried to the back of the mucoperiosteal incision.
The throat pack is then removed. The Dingman mouth gag is also removed. The patient's stomach, esophagus, and nasopharynx are gently suctioned.
Successful surgical outcomes in cleft palate repair are directly linked to the extent of mobilization of the palatal tissues. [26] Excessive tension or convergence of more than 2 flaps may predispose repairs to dehiscence or fistulization. Operative variations that may affect ultimate speech results include the following:
-
Repositioning the levator palatini muscles
-
Use of flaps from the nasal floor
-
Vomer flap closure of the hard palatal cleft, if necessary
-
Buccal sulcus flap for closure of the alveolar cleft
The 2-flap palatoplasty technique popularized by Salyer and Bardach is still widely used for primary palatoplasty. The procedure is described as follows:
-
Incise the medial edges of the cleft, extending anteriorly to the uvular halves.
-
Make a relaxing incision just anterior to the anterior tonsillar pillar, around the posterior maxillary tubercle, and then forward to approximately the level of the premolar teeth.
-
Elevate mucoperiosteal flaps from the hard palate, isolating the greater palatine vessels and stretching them out of their foramen.
-
Dissect medially from the pterygoid hamulus along the posterior edge of the hard palate.
-
The hamulus can be fractured medially without interfering with eustachian tube function, yet most of the time this maneuver is unhelpful and rarely necessary.
-
Pick up the edge of the cleft mucosa with a single hook and dissect posteriorly from the bony surface at the posterior edge of the hard palate between the oral mucosa and levator muscle. This maneuver demonstrates the longitudinal position of the levator palatini muscles and the abnormal insertions on the bony palate along the medial portion of the bony cleft.
-
These maneuvers expose the vertical orientation of the muscle origin towards the base of the brain.
-
If performing intravelar veloplasty (IVV), separate the muscular insertions from the bone and begin dissection between the muscle and nasal mucosa.
-
The levator muscle is then completely mobilized for repositioning into a transverse location and is overlapped to re-create the levator sling.
-
The nasal mucosa may be lengthened, either with a Z-plasty or by raising flaps from the nasal floor.
Does use of a vomer flap interfere with maxillofacial growth?
Freide and Johanson believe it does, [27] while other authors who have used this technique for closure of the hard palate find it causes no significant growth disturbance when performed synchronously with lip repair. According to the Oslo Cleft Palate Consortium, the vomer flap provides effective and early closure of the alveolar cleft and prevents the risk of fistula formation, while causing no significant untoward influence on facial growth.
A study by de Jong and Breugem also concluded that lip closure accompanied by early hard palate closure using a vomer flap results in a significant reduction of the residual cleft when compared to lip closure alone. [28]
What can be done at primary palatoplasty to improve velopharyngeal function?
For many years, several authors supported performing an IVV to improve velopharyngeal function. This procedure directly reconstructs the abnormally inserting velar muscles. No comparative studies have established whether it is truly effective. The only prospective study extant suggests that speech improvement is not significant enough to recommend routine performance of an IVV.
What should be done when an alveolar nasal fistula is present?
Some cleft care providers do not advocate treatment of an alveolar nasal fistula; however, this procedure may enhance both cosmesis and hygiene by preventing nasal regurgitation, malodor, and rhinitis. Additionally, even a very small fistula can interfere with normal speech. Management of the alveolar nasal fistula also provides the benefit of unscarred tissue to be used for later alveolar bone grafting.
When Does the Requirement of Surgical and Nonsurgical Therapy Decrease?
The presence of a congenital facial cleft requires a long-term treatment plan. Initial evaluation and management is undertaken soon after birth and continues, to some degree, into early adulthood. Many secondary problems require attention in adolescence. Additional problems that may persist beyond school age or into early adulthood include the following:
-
Deviated nasal septum (airway obstruction)
-
Hearing impairment
-
Recurrent ear infections
-
Malocclusion
-
Abnormal craniofacial growth
-
Inability to generate a pressure gradient between the oral and nasal chambers (hinders sucking in most infants)
-
Speech dysfunction: This is the most serious untoward consequence associated with cleft palate; in most instances, children experience hypernasality or escape of sound into the nasal cavity associated with the production of many consonant phonemes and vowels in the English language, except m, n, and ng.
-
Funding: In many states, funding for cleft care extends until the patient is aged 21 years. An individual affected with an orofacial cleft would only rarely require major interventions beyond this time.
Missions to Developing Countries
Worldwide, approximately 250,000 infants are born each year with cleft lip and cleft palate. Facial appearance and speech are fundamental human needs, critical for social interaction, communication, and quality of life. In many developing countries, individuals with orofacial clefts are untreated. This unfortunate circumstance is due to a combination of unavailability of surgical services, lack of organization, inadequate funding for equipment and technology, and lack of focus on conditions that are not life-threatening. As a result, many organizations worldwide have reached out to provide some of the unmet needs by way of short- or long-term "medical missions."
Some humanitarian organizations have fallen under criticism for various reasons. For example, some organizations have not used qualified local surgeons or have replaced them with volunteers who are not qualified or expert in cleft management. Other criticisms have been directed at missions that have used the outreach venue as a "training ground" for resident experience. The American Cleft Palate Craniofacial Association has developed a consensus position that focuses on training local professionals, insisting on medical records, providing adequate preoperative screening and postoperative patient follow-up, and limiting the number of operations performed on a given outreach project.
What Resources Are Available to Parents?
The following links provide information for parents of patients with cleft palate:
-
Two morphologically distinct parts comprise the secondary palate, soft tissue (posterior), and bony palatal components. Note the transverse orientation of the levator muscles. Also note the position of the incisive foramen separating the anterior structures, which are involved in the prepalatal (primary) palatal clefts, compared with the posterior structures, which are involved in the palatal (secondary) palatal clefts.
-
Embryology processes of abnormal palatal development. Normally, the palatal shelves assume a vertical orientation and are directed downward. Free communication exists between the oral cavity and the nose at 7 weeks. Thus, the tongue is in direct contact with the inferior border of the nasal septum. The palatal shelves move horizontally as the process of fusion commences. Failure of fusion of the palatal shelves (medial extensions of the maxillary prominence), from any cause, may lead to cleft palate.
-
Development of the secondary palate. The process is initiated when outgrowths of the palatal shelves push medially and bilaterally from the maxilla.
-
Palatal shelves grow downward and adjacent to the tongue.
-
Palatal shelves assume a level above the tongue and assume a horizontal position. Fusion of the 3 processes forms the normal secondary palate.
-
Spectrum of cleft palate morphologies (from upper left panning to right). A. Normal development. B. Unilateral clefting of the lip. C. Unilateral clefting of the lip and primary palate. D. Bilateral clefting of the lips and primary palate including the incisive canal. E. Secondary palate cleft including the incisive canal. F. Unilateral clefting of lip, primary palate, incisive canal, and secondary palate.
-
The left illustration is of normal soft palatal anatomy. Notice the transverse orientation of the levator muscles across the posterior portion of the soft palate. The right illustration shows cleft anatomy, in which the levator muscles are orientated more longitudinally and insert on the posterior edge of the palatal bone and along the bony cleft edges.
-
Millard modification of Kernohan striped-Y classification for cleft lip and palate. The small circle indicates the incisive foramen; the triangles indicate the nasal tip and nasal floor.
-
Illustration of Furlow technique, first step.
-
Illustration of Furlow technique, second step.
-
Illustration of Furlow technique, third step.
-
Illustration of Furlow technique, fourth step.
-
Animation of face development.
-
Animation of palate development.
-
Animation of palate development.
-
Animation of palate development.
Tables
What would you like to print?
- Cleft Palate
- Cleft Palate Appearance
- How Does Cleft Palate Affect Hearing and Speech?
- What Are the Key Anatomic Features of the Palate?
- When Should a Cleft Palate Be Repaired?
- When Does the Requirement of Surgical and Nonsurgical Therapy Decrease?
- Missions to Developing Countries
- What Resources Are Available to Parents?
- Show All
- Media Gallery
- References