Overview
A great deal of safety research combined with decades of clinical experience has proven the efficacy and relative safety of silicone gel breast implants. Aside from the unique adverse effect of capsular contracture, the complication rate of silicone implant surgery approaches that of any clean elective procedure. To date, no convincing evidence exists of any systemic disorder attributable to silicone.
As these are artificial devices, they have a failure rate and, in some patients, can require a significant amount of surgical maintenance. As with all natural body parts, these artificial substitutes may be subject to injury or disease and, when viewed from that perspective, have favorable risk-benefit characteristics.
Glossary
Silicone and related substances are defined as follows:
-
Silicon - Silicon is a metal in the same column as carbon in the periodic table; it is the most abundant element on earth and does not occur naturally in its pure metallic state
-
Silica - Silica in its crystalline form exists as common sand, marble, or quartz; it also occurs in an amorphous form, and very fine, extremely pure, amorphous silica is used as a filler to strengthen solid silicone, such as in the shell of an implant (each grain of silica is encapsulated tightly in silicone so that even when the elastomer is abraded or torn, no silica is exposed to the body)
-
Silicate - In one form, the hydroscopic properties of silicate are used to keep the contents of containers dry
Dimethylsiloxane is the building block for most medical-grade silicone products, including breast implants. It can be made extremely pure and modified into products with a multitude of characteristics.
Surgical procedure
Surgery for insertion of the devices can be performed under local or general anesthesia and is usually an outpatient procedure. The incision for cosmetic insertion most commonly is made along the lower edge of the areola, in the axilla, or in the inframammary fold. For postmastectomy reconstruction, the existing surgical scar usually is used for access. [1]
A generous pocket is made for the implant in a plane either deep to the breast on the pectoral fascia (submammary) or beneath the pectoralis major and/or serratus fascia (submuscular). The implant is then inserted and the incision closed.
All of the normal risks of surgery and anesthesia can occur with breast augmentation or reconstruction. [2] Infection, bleeding, change in nipple sensation, malposition (hyposensitivity, hypersensitivity), poor healing, anesthetic accidents, and other complications can occur at a rate that is similar to that in any clean surgery in this area on healthy patients. Concerns specific to breast implantation, including repeat surgery and capsular contracture, are discussed later in the article.
History of Breast Implants
The number of women in the United States who have breast implants is unknown, but current estimates derived from national surveys range up to more than 6 million. This represents more than 5% of the adult female population. The American Society of Plastic Surgeons (ASPS) collects information annually on plastic surgery procedures performed by its members. In 2008, approximately 307,000 women received breast implants for cosmetic breast augmentation and 111,000 for reconstruction of congenital or postmastectomy deformities. [3] These data do not include those procedures (mostly cosmetic) performed by doctors other than plastic surgeons, such as otolaryngologists, general surgeons, gynecologists, and others.
Following adverse publicity in the early 1990s, interest in the breast implant procedure fell significantly, especially for gel-filled devices, but it seems to have recovered incrementally. According to the ASPS, in 2008, 53% of total breast implants were filled with saline and 47% were filled with silicone. [3, 4]
Prior to 1963, various plastic foam materials were used; for breast augmentation, however, it became apparent that the air cells would collapse and, combined with tissue ingrowth, shrink and harden the device. These materials were wrapped in plastic film to minimize this effect, but to no avail. Amazingly, however, some of these implants were so well tolerated that they have stayed in place to this day.
The modern silicone breast implant has been available since 1963 and has gone through an evolution of change and improvement. Several types of devices, with many variations, shapes and styles within each class, are now available or under testing for US Food and Drug Administration (FDA) approval. Basic to all implants is a silicone rubber (elastomer) shell, which can be single or double, smooth or textured, barrier coated, or covered with polyurethane foam. The foam-covered devices have not been available in the United States since 1990 but are still marketed in Europe.
The contents are either factory-filled with silicone gel of various consistencies or inflated at surgery with normal saline. One brand that was manufactured overseas was prefilled with saline at the factory. It was briefly marketed in the United States but was later withdrawn when the FDA denied approval.
The double-lumen devices consist of concentric balloons that contain silicone in one chamber and saline in the other. The only one still in use is the Becker, which has an outer layer of gel and an inner balloon that is valved to permit gradual, postoperative inflation with saline. This is termed a "permanent tissue expander," since it permits gradual and temporary overinflation to create the pocket and then can be left in as a permanent implant after the size is adjusted appropriately. At this writing, the Becker devices are not generally available in the United States pending evaluation of FDA mandated studies. [5]
In 1990, the FDA placed a moratorium on the use of gel-filled implants for cosmetic augmentation. The implants remained available for reconstruction and replacement, but extensive record keeping, follow-up, and institutional review board (IRB) approval were required for their use. In 2006, after extensive study and analysis, the FDA deemed the implants safe for all augmentation and reconstructive purposes, but patient tracking is still required. [6]
Saline implants
Saline filled implants are available as empty, balloonlike silicone devices to be filled with normal saline at the time of surgery. This permits subtle size adjustments to compensate for asymmetry between the breasts. They are less popular than silicone implants, as they often may have a less natural feel. If the patient has very little breast tissue or only a skin covering after mastectomy, unsightly wrinkles and folds of the device may be visible on the breast. This is more common when the surface is textured.
Silicone gel implants
Three generations of basic design of this device have been created, with many variations within each type.
First generation
The first models to be marketed had envelopes of thick, smooth-walled silicone elastomer made in 2 sections, which were filled with a viscous silicone gel material (dimethylsiloxane) and glued together. They were available in only 3 sizes: small, medium, and large.
In the first few years, surgeons believed that the device required attachment to the tissues to prevent migration. Scar ingrowth for fixation was accomplished by patches of material (eg, Dacron mesh or perforated silicone) attached to the back of the device or by an outer covering of polyurethane foam. The Dacron and silicone patches were subsequently found to be unnecessary and actually detracted from the quality of the result. Some patches or tabs created a stress point that led to tears of the envelope. Fixation patches were eliminated in the early 1970s.
Second generation
Manufacturers varied the gel consistency and shell thickness in an attempt to improve performance. Beginning in the mid-1970s, the shells were made thinner and the gel less viscous (ie, more "responsive"), primarily in an ill-conceived attempt to control hardening from scar shrinkage (capsular contracture.) This trend reversed in the early 1980s when it was recognized as ineffective in reducing contracture and as resulting in a more fragile device. After 10 years, most of these implants had broken.
Third generation
New formulations of the shell and gel contents became available that were stronger and had a second barrier coat of diphenyl silicone. This coating almost totally eliminated so-called "gel bleed" or diffusion of small amounts of the silicone oil through the implant shell. The gel content also was made more viscous and cohesive.
In 1989, textured-surface shells that many surgeons hoped would minimize the incidence of unwanted firmness from capsular contracture became available. However, studies have been somewhat confusing regarding whether this was effective. The textured implants had the disadvantage of a higher rupture rate than the more traditional smooth shells and often produced visible wrinkles in the breast in women with very little overlying tissue to mask the ripples. Because of these shortcomings and a lack of solid evidence that these devices are softer, they have become much less popular.
Polyurethane-covered implants
In the late 1960s, a variation of the breast implant was developed containing a polyurethane sponge coating over an otherwise standard gel-filled implant. Although the coating originally was planned as a fixation layer, many surgeons came to believe that the foam cover resulted in a decreased incidence (or at least a delayed onset) of capsular contracture. These implants also evolved in shape and design, culminating in the early 1980s with the Meme and Optimam styles. In April 1991, the manufacturer voluntarily withdrew the foam-covered implants from the market. [7]
One style, the MemeME, had a unique construction. It had no true shell, but a skin of sorts was formed in situ by spraying the surface with silicone containing extra catalyst prior to curing. This increased the crosslinking of the surface to create a shell-like membrane. The polyurethane foam was then shaped and sealed over the surface. Implants of this particular type were known to occasionally extrude some of their gel contents through the foam when squeezed. This is a possible explanation for reports of blood being found within the substance of the gel in apparently intact implants. The MemeME model was marketed from 1983-1988.
Other filler materials
While silicone remains the only available shell material, other filler substances have been used in Europe and South America and, at one time, were under development or in experimental trial in the United States. Various hydrogels and a pure form of triglycerides were the 2 main formulations.
The major advantage of the triglyceride formulation (Trilucent) was that it had a Z number (measure of radiolucency) similar to that of fat, thus resulting in little or no compromise of mammography. Another fill substance, polyvinyl pyrrolidone in saline, was briefly available, under the trade name Misty Gold. None of these products are currently available in the United States. At this time, only silicone gel or saline-filled models are available for use in the United States.
Current implants
In late 2006, a new formulation of silicone gel filler called MemoryGel (Mentor Worldwide LLC) gained FDA approval. [8] This gel implant is thicker and more cohesive so as to minimize gel spread in the case of rupture and to resist scar shrinkage that would deform its contour. When cut, the gel retains its shape and doesn’t run. This device has a doughy feel to it.
Currently available devices in the United States are saline- or silicone-filled implants with either textured or smooth surfaces. They come in round or teardrop shapes with a choice of three different projections. Companies with FDA approval to market these devices in the United States include Allergan Aesthetics, Ideal Implant Inc, Mentor Worldwide LLC, and Sientra Inc. [9]
The implants produced currently are much improved devices compared with earlier units. The shell is still made of an outer layer of a mix of dimethyl siloxane and amorphous silica with an inner barrier coat of diphenyl siloxane to minimize silicone gel bleed. The shells, on testing for breakage, exceed the American Society for Testing and Materials (ASTM) requirements by more than 300%. The gel is more cohesive, varying from a standard 60% crosslinking to 80% for the more cohesive type nicknamed "gummy bear" (which has a consistency similar to the candy).
As evidenced by sales figures prior to the moratorium and those following the subsequent release of gel-filled implants for cosmetic purposes, approximately 80-85% of surgeons and patients prefer the quality of results obtained by gel implants, making them the implants of choice. In 1997, sales figures for Europe, where usage was unrestricted, showed a distribution of 70% for gel, 15% for saline, and 15% for alternate fills such as triglycerides (then still available) and hydrogels for cosmetic use.
Safety
Silicone is probably the most studied implantable material available today. After over 35 well-conducted studies from many countries, it seems certain that this material does not cause disease. The results of more than 7 long-term follow-up studies showed that women with implants have a reduced incidence of breast cancer than is otherwise expected in the general population.
No hard evidence reveals that a broken implant is harmful. Almost all of the problems that can occur with breast implants, such as infection, hardening, extrusion, and malposition are related to the surgical procedure or the patient's own biology, not the device.
Benefits
Many social subsets of women with various motivations seek breast implants, especially for cosmetic purposes. However, the prototypical recipient is in her early 30s and is secure and successful in most of her activities, except for this single focus of concern. She is fully aware of the unique ambivalence that society and often family and friends have to an artificial bosom (ie, that breasts are sexy and attractive if natural but somehow frivolous and vain if sought through surgery).
Thus, in contrast to the common cliché, these women seek augmentation despite, rather than because of, social pressures. The depth of this personal need and the importance of this procedure to their sense of wholeness and self-esteem are difficult for even their loved ones and their personal physicians to appreciate. Only the woman and perhaps the plastic surgeon who hears these stories over and over again can understand the power of this need and the significant enhancement of quality of life that these devices provide.
Several surveys consistently have demonstrated that 90-95% of women who have undergone cosmetic augmentations are pleased that they did so, even if the results were less than ideal or were accompanied by complications. At the height of negative media information about implants in 1991, a survey of 300 plastic surgeons revealed that approximately 3% of women who made inquiries about removal of their gel-filled implants did so due to safety concerns. Less than one half followed through, which is a measure of the great value of this operation.
This estimate is similar to the percentage of women who took advantage of the implant manufacturers' offer of financial support for implant removal or replacement. Plastic surgeons suspected that the number of requests for removal of gel devices, usually with saline replacement, increased commensurately with the publicity over the multibillion-dollar class action settlement.
The desire of most women to replace their implants with saline (76% according to a study by Spear and Bowen) reflects their satisfaction with the enlargement. As reassuring research on safety has become available, interest in removal appears to have fallen significantly. Current estimates suggest that 85% of removed implants are replaced. Some women, having experienced both gel and saline, are requesting a return to the gel, feeling that it provided a superior result.
The number of cosmetic breast augmentations now appears to exceed premoratorium estimates following the significant dip in procedures during the early and mid-1990s. (See Table 1, below.)
Table 1. Annual Implant Sales by Pairs (Open Table in a new window)
1990 |
120,000 |
|
1991 |
110,000 |
|
1992 |
60,000 |
|
1993 |
76,000 |
|
1994 |
84,000 |
|
1995 |
99,000 |
|
1996 |
118,000 |
|
1997 |
ASPS |
122,000 |
ASAPS |
101,000 |
|
Manufacturers |
230,000 |
|
1998 |
ASPS |
132,000 |
ASAPS |
126,000 |
|
Manufacturers |
300,000 |
|
1990-1996 figures represent manufacturer's estimates for all implant sales, including augmentation, reconstruction, and replacement. American Society of Plastic Surgeons (ASPS) and American Society for Aesthetic Plastic Surgery (ASAPS) data represent separately collected and analyzed membership statistics on cosmetic augmentation using different methodologies. These do not include patients of nonmembers, which is substantial. All numbers are approximations. |
||
Contraindications
Aside from the usual medical conditions that would increase risks from anesthesia or surgery or increase the risk of infection, the most significant red flag, as in all cosmetic surgery, is an unrealistic expectation. Women who are emotionally unstable or are requesting the surgery to please another person should be discouraged from undergoing the procedure. However, this should not be considered a blanket contraindication.
Each patient must be individually evaluated and a decision made as to whether the procedure will enhance the quality of her life, her sense of self, and her sense of well-being. Plastic surgeons must develop the skills to evaluate these intangibles and take the time for a proper evaluation. Because it is so subjective, do not expect 100% accuracy in predicting the outcome; guessing correctly 95% of the time is the best that can be expected.
Properties and Uses of Silicone
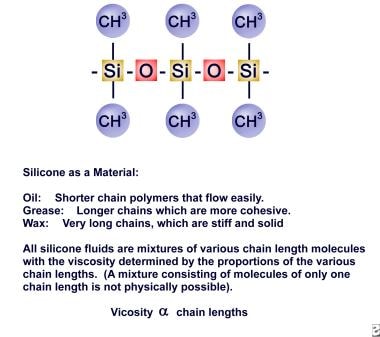
Keep in mind the difference between elemental silicon and the polymer silicone. Medical-grade silicone is usually a specific, very pure polymer of silicon and oxygen with methyl side groups (dimethylsiloxane). It is one of the least bioreactive materials available for use in medical devices. The breast implant shell is made of a rubberlike membrane of fully polymerized silicone with an amorphous (noncrystalline) silica filler added for strength.
Until the moratorium, most implants used were filled with a silicone gel, the physical form of which can be likened to a spongelike matrix or 3-dimensional (3-D) net filled with various chain-length silicone oils. These form a physical chemical bond resulting in a gel. The shell membrane is slightly permeable to the oils.
Depending on the brand, age, characteristics, and environmental mechanics of a particular device, small amounts of the oil diffuse or bleed through the shell. For most implants, this is a matter of a few grams. Newer barrier coat devices introduced in the early 1980s bleed at as little as one tenth the rate of the older materials.
This leakage of silicone should be viewed in perspective. Medical-grade silicone is ubiquitous in the environment, and probably everyone in the civilized world has some form of silicone in his or her body. For example, every disposable needle and syringe, as well as intravenous tubing, is lubricated with silicone. (The FDA permits up to 1mg/cm2 of barrel surface.)
Medical uses of silicone
Medications in stoppered vials contain residual silicone from its use in the manufacturing process. Silicone is hydrophobic and lipophilic; thus, various amounts may be injected along with the medication depending on the lipid characteristics of the drug used. Because insulin binds to silicone, extrapolative calculations suggest that patients with type I diabetes may inject as much as 25-30 g of silicone over a lifetime.
In its solid form, silicone elastomers are used for pacemaker coatings, tubing, prosthetic joints, hydrocephalus shunts, and penile implants, and as the envelope for Norplant and other implanted drug-delivery systems. Some testicular and chin implants are similar to breast implants, since both usually are made of a silicone gel in a silicone envelope.
More than 1000 medical products contain silicone as either a component or as a residuum from use in the manufacturing process. Silicone is a nonspecific term for a class of compounds, some of which are highly reactive or toxic. The generic term silicone is similar to the generic term oil, which can include salad oil and motor oil. Within the subclass of medical-grade material, the formulations vary to some degree with intended use. The body may react differently to some of these formulations.
Methicone
The designation methicone (as in simethicone or dimethicone) as an ingredient in any medication is simply silicone formulated to comply with FDA regulations for human consumption in items such as medication, foods, and cosmetics. Calcium carbonate, magnesia, simethicone antacid (Di-Gel), and oral simethicone (Mylicon) are examples of medications containing silicone that are marketed over the counter, even in pediatric formulations, with FDA approval. Silicones are used in lipstick, hairspray, food processing, skin creams, and cosmetics and are known to be absorbed through both the bowel and the lungs.
Biologic reactions
Biologically, medical-grade silicones invoke a straightforward, nonspecific foreign body response, resulting in typical macrophage invasion, giant cell formation, and eventual scarring. Several animal studies suggest that relatively huge volumes of gel injected into the peritoneal cavity of rodents may stimulate an immune response. This is not observed with the oil or solid elastomers. It can be demonstrated only by emulsifying the gel, a condition not seen in the implant. Intact gel does not lend itself to these test procedures.
Exhaustive evaluations by multiple prestigious scientific bodies such as the Institute of Medicine, the British Ministry of Health, the Spanish government, EQUAM (the European Committee on Quality Assurance and Medical Devices in Plastic Surgery), Harvard University, the Mayo Clinic, and multiple panels of experts established by various courts have confirmed that no evidence exists of any known or new systemic illness that can be definitively attributed to silicones. Indeed, more than 30 quality epidemiologic studies of the health consequences of implants have been performed, and none can find any evidence of health risk.
Silicone-related neurologic disorders
A few physicians have claimed that neurologic disorders such as multiple sclerosis and amyotrophic lateral sclerosis can result from silicone "toxicity." These reports have surfaced mostly in the courtroom with very little reaching mainstream medical literature.
Silicone-related teratogenicity and mutagenicity
In the few animal studies published to date, no evidence of fetal abnormalities has been demonstrated. One publication anecdotally reported gastrointestinal (GI) disturbances in 9 children of mothers with implants, but most experts are unconvinced by the connection. A later publication by some of the same authors showed no difference in autoantibody production between children with GI disorders born to mothers with implants and those born to mothers without implants.
Platinum-related sensitization
Organic platinum compounds such as platinic acid are used in tiny amounts as polymerizing agents in the fabrication of silicone materials acid and are considered benign. However, as an inorganic salt, platinum can be one of the most sensitizing chemicals known. Claims have been made in the courtroom incorrectly attributing the same sensitizing effect observed with inorganic salts to the organic form.
Repeat Surgery
Most women who have implants achieve satisfactory results from 1 operation, and the implants remain indefinitely without difficulty. As many as 20% of women may need repeat surgery (often more than once). Reoperation may be required for a host of reasons, and for a few women, these devices become high-maintenance items. To place this in perspective, do not expect more from an implant than from any natural body tissue or organ. These devices can be thought of as new body parts and, like our own organs, they may last a lifetime or cause frequent difficulty throughout life.
A retrospective study by Khan in a single-surgeon practice indicated that with regard to the use of round silicone breast implants, the periprosthetic infection rate does not significantly differ between primary and secondary augmentation mammoplasty (0.7% and 0.5%, respectively). The investigator did find, however, that in primary augmentation, wound healing and superficial skin issues were greater than in secondary augmentation by a statistically significant margin. [10]
Capsular Contracture
Often referred to as a complication, this phenomenon is best considered an adverse effect. It is the result of the normal process of scar formation resulting from the repair of separation of tissue. (One would not consider the skin scar to be a complication of a cut, despite the spectrum of final appearances.)
Shrinkage or shortening of a scar is a poorly understood phenomenon that varies dramatically among individuals and at different locations and directions in the same person. Contracture around an implant is probably an aborted attempt at extrusion of a foreign body. In truth, the mystery is why contracture does not occur in everyone.
Contracture is the most common adverse effect of breast implants. To achieve a soft, natural-feeling result, the surgical pocket is made somewhat larger than the implant. Normal wound healing forms a scar lining on the pocket surface termed the capsule, which, under ideal circumstances, retains its original dimensions. The oversized pocket permits full flexibility of the implant, often resulting in a breast so soft that the implant is not palpable and closely mimics normal breast mobility and softness.
For reasons that are unclear and appear to be related to a particular woman's individual biology, the scar envelope sometimes shrinks and squeezes the implant, producing varying degrees of firmness. This commonly is graded on a scale devised by Baker, as follows:
-
Grade I (none) - The augmented breast feels as soft as an unoperated breast
-
Grade II (minimal) - The breast is less soft; the implant can be palpated but is not visible
-
Grade III (moderate) - The breast is firmer; the implant is felt easily, and its presence is visible
-
Grade IV (severe) - The implant is firm and often tender, painful, cool, and distorted; its presence is obvious
Contracture can occur soon after surgery or many years later and may be symmetrical, asymmetrical, or unilateral. Current theory suggests that low-grade contamination by Staphylococcus epidermidis may be the initiating factor of the contracture, but this is not confirmed.
Capsular contracture does not in itself pose a health risk, aside from the attendant risks of surgical correction, if required, or the fact that the contracture may interfere with mammography. However, contractures detract from the quality of the implants' results, with the severity of concern depending on the individual patient. The best results achieve the ideal of a breast so soft that the implant is undetectable. Yet many women prefer a slightly firm bosom while for others even a severe contracture is only a minor nuisance.
The amount of overlying breast tissue as padding influences the sense of softness and the appearance. If the tissues are tight, usually the breast has a superior fullness similar to the effect of a push-up bra. The most severe degree of contracture may be unaesthetic or deformed, quite uncomfortable, or chronically painful.
Texturing
The innovation of texturing the implant shell initially showed promise in reducing the incidence of contracture. However, data from both US manufacturers have been confusing but suggest that little or no difference exists for saline implants. In contrast, the most recent information concerning gel implants demonstrates statistically less contracture in cosmetic patients with the textured variety (smooth 15%; textured 9%) but suggests that texturing does not make any difference in reconstruction (smooth 13%; textured 12%).
An undesirable adverse effect of texturing is an unpleasant rippling of the breast surface, especially — as can be the case in very small breasts — if little overlying tissue padding is present. This may be visible and unsightly or just palpable and annoying, depending on the thickness of normal breast tissue and subcutaneous fat available to mask the irregularities. For most women, firmness is a more acceptable compromise than rippling, especially if it is in the cleavage area.
Carcinogenesis
Breast cancer
No evidence exists that the silicone used in breast implants is carcinogenic in humans. More than 7 epidemiologic studies confirm this, at least for the 10- to 30-year periods covered by these reports. [11, 12] Moreover, 3 large-scale studies and 2 smaller ones have demonstrated that women with implants may have up to 30% less breast cancer than expected statistically when matched with the general population.
Three animal studies show the same protective effect, and 1 preliminary report suggests that blood from women with implants kills breast cancer cells in tissue culture. Therefore, all evidence suggests that at least for the greater than 30-year time frame that silicone implants have been available, the risk of humans developing cancer from silicone breast implants is negligible, if not nil. Studies also demonstrate that 5-year survival rates for breast cancer patients are not affected by the presence of these devices. [13]
Myeloma
A study from the National Institutes of Health (NIH) demonstrated the development of myelomas in susceptible strains of rats injected with gel in their peritoneal cavities. This was not observed with oil or elastomer. Eighteen cases are now in a newly established registry at NIH. They are clustered in Los Angeles, Arkansas, and Florida (no new cases have been added since the first 18 were collected 4y ago).
Monoclonal gammopathy of undetermined significance (MGUS) is found in the serum as a monoclonal immunoglobulin G (IgG) or IgA in 1.5% of the otherwise healthy population. This is believed to have some predictive value in determining the risk of myeloma, since 16% of those with elevated levels develop the disease within 30 years (0.8%/y). A small sample of women with implants has demonstrated an elevated MGUS fraction. However, a search through 4 registries containing more than 20,000 women with more than 120,000 years of risk located only 1 case of myeloma. This information should be considered and studied further, but the evidence is too sketchy to generate alarm. A statistical truism is that "an association does not imply a cause-and-effect relationship."
Anaplastic large T-cell lymphoma
Anaplastic large T-cell lymphoma (ALCL) of the breast has been identified as having a potential association with breast implants. [14] By April 1, 2022, there had been 1130 US and global medical device reports of breast implant–associated ALCL (BIA-ALCL) received by the FDA. [15] Fortunately, this disease is not only rare but can often be treated with less complexity than primary breast cancer. Current recommended treatment for BIA-ALCL is capsulectomy and implant removal.
The risk for ALCL has been found to be higher with textured breast implants than with smooth ones. [16] In a survey sent to active and candidate members of The Aesthetic Society (470 responses), Frojo et al determined that with regard to patients with textured implants who are asymptomatic but have concerns about the lymphoma, 11.2% of surgeons recommend that the implant be removed, while 88.8% respond by educating the patient, with clinical follow-up and/or imaging included. [17]
In 2019, at the FDA’s request, Allergan issued a voluntary recall of its Biocell textured breast implants and tissue expanders, with the FDA having found evidence that the risk of BIA-ALCL was about six times greater with the recalled implants than with other textured implants being marketed in the United States. [18]
Guidelines
Consensus statements from Italy, developed by a multidisciplinary team, provide recommendations on the diagnosis and management of BIA-ALCL. The statements include, but are not limited to, the following. [19]
For patients with stage I BIA-ALCL [19] :
-
The gold standard of care is implant removal and en-bloc capsulectomy
-
If, following oncologic surgery, there is evidence of negative resection margins (R0), patients should undergo clinical-radiologic follow-up evaluation every 3-6 months for the first 2 years and then annual follow-up for up to 5 years
For patients with stage II BIA-ALCL [19] :
-
Adequate treatment planning requires multidisciplinary team evaluation
-
It is recommended that surgically, patients undergo implant removal, en-bloc capsulectomy, and complete resection of the tumor mass; for patients who have locally advanced disease with lymph node involvement (stage IIB T1-N1M0), surgery should also include lymphadenectomy
-
Adjuvant chemotherapy and immunotherapy should be provided to patients whose disease is localized to the capsule and has lymph node involvement (ie, stage IIB BIA-ALCL [T1-3N1M0])
For patients with stage III BIA-ALCL [19] :
-
Adequate treatment planning requires multidisciplinary team evaluation
-
It is recommended that surgically, patients with stage III BIA-ALCL (T4N[1-2]M0) undergo implant removal, en-bloc capsulectomy, complete resection of the tumor mass, and lymphadenectomy
-
Adjuvant chemotherapy and immunotherapy should be provided to patients with BIA-ALCL extending beyond the capsule and with lymph node involvement (ie, stage III BIA-ALCL [T4N(1–2)M0])
For patients with stage IV BIA-ALCL [19] :
-
Adequate treatment planning of stage IV BIA-ALCL (AnyT; AnyN; M1) requires multidisciplinary team evaluation
-
With prospective trials lacking, “therapeutic management of disseminated stage IV BIA-ALCL should be guided by current experience and protocols utilized in the treatment of ALCL lymphomas”
-
In disseminated stage IV BIA-ALCL, tumor debulking surgery should be considered as initial treatment approach
Polyurethane-Coated Implants and Cancer
The polyurethane coat that covered some implants in the United States before 1991 is known to slowly hydrolyze over several years into unknown breakdown products. Of concern is one constituent of the polymer, 2, 4, toluene diamine (TDA). In studies performed in the 1960s, large doses of TDA fed to highly cancer-prone rats produced hepatomas. When viewed in the light of a more modern understanding, the validity of these early experiments is questionable.
TDA never has been documented to be a human carcinogen, but because of this animal evidence, current law, known as the Delaney clause, requires that it be banned by the FDA for use in foods, cosmetics, and implantable devices. (Congress amended this clause to make it more pertinent to scientific reality.) However, no evidence exists that TDA is formed in vivo from polyurethane. While TDA is a building block of polyurethane, it is not produced by hydrolysis, since the molecule is cleaved at the urea fraction rather than at the TDA site. An FDA advisory panel hearing held in July 1991 concluded that the probable cancer risk from polyurethane-coated implants is considered negligible (< 1 in 1,000,000).
Studies subsequently demonstrated, however, that approximately 80% of women with polyurethane-coated implants showed traces of TDA in their urine. Traces of TDA also were found in the urine of 11% of control subjects, suggesting an environmental source of the chemical. None was found in the blood of patients or controls. Again, the risk of cancer from polyurethane implants was calculated to be less than 1 in 1 million.
To date, no cases have been reported in the medical literature of cancer in a woman with polyurethane implants. Because the small, but real, risks associated with implant removal are greater than the risk of cancer, the FDA has advised that there is no health-related reason that requires removal of these particular devices in asymptomatic women.
Breast Cancer Detection Concerns
Perhaps the most significant concern regarding breast implants is the possibility of delayed detection of breast cancer. The implant itself is radio-opaque and variably compresses the breast tissues, depending on the particular configuration of a woman's breast architecture and the degree of contracture. A real concern involves the presence of an implant compromising mammography and delaying detection until the mass is large enough to be palpable. Several studies have shown that this appears to be only a theoretic risk, since the stage of detection of the breast cancer in women with implants appears to be identical or better than that of the overall population. [20, 21]
Mammographic techniques have improved dramatically, to the point that the mammographer can minimize the amount of breast that is hidden by an implant. Approximately 9% to more than 20% of breast cancers in all women are invisible on radiographs. Despite the increasing use of mammography, most breast cancers still are discovered by self-examination or physician examination. Many clinicians believe that the presence of an implant can increase the ease of palpation. To date, no cases have been documented in the medical literature in which a diagnosis of breast cancer was delayed by the presence of an implant. This suggests that this is a rare occurrence, at worst.
The Society for Breast Imaging, the American Society of Plastic and Reconstructive Surgeons (ASPRS), and the American Cancer Society agree that a woman with breast implants should be on the same schedule of routine mammography as other women, as follows:
-
Baseline - At the earliest when aged 35 years
-
Biannually - When aged 40-50 years
-
Annually - When older than 50 years
However, a woman with breast implants should avoid screening clinics where only 2 routine views of the breast are taken. [20, 22] Instead, she should be referred to mammographic units accredited by the American College of Radiology, which are familiar with the special displacement (Eklund) views required for proper mammographic evaluation of the implanted breast. [23, 24, 25] Ideally, the woman should try to obtain her studies at the same facility each time so that her films can be observed serially, and she should inform the technician that she has implants. Because extra views are required, the cost of the mammogram for patients with breast implants is modestly higher.
Preoperative mammograms in women younger than 35 years should be discouraged. Cancers can be observed best when the breast parenchyma is mostly fat. The young breast has dense stroma, thus mammography contributes little or nothing to diagnosis of any breast disease. In addition, evidence exists that the young breast is much more vulnerable to radiation damage.
For patient education information, see the Women's Health Center, as well as Breast Lumps and Pain.
Rheumatologic Disorders
The media and the courtroom have made much about autoimmune disease, human adjuvant disease, or silicone-associated disorder (SAD). Almost every disease and symptom complex from scleroderma to chronic fatigue syndrome to multiple sclerosis and amyotrophic lateral sclerosis has been blamed on the breast implant in various anecdotal reports. More than 200 separate conditions have been listed in plaintiff pleadings as having been caused by these devices. The College of Rheumatology, American Medical Association, and FDA agree that the term human adjuvant disease is inaccurate and inappropriate. These disorders, if they exist, should be labeled simply rheumatologic or immune disorders.
Of those rheumatologic disorders most likely to be caused from exogenous sources, scleroderma (sclerodermalike syndrome is the correct term for the variants that arise from exogenous toxins), lupus, and Sjögren disease are the most probable candidates. Scleroderma is a rare disease with an estimated incidence of approximately 10,000 new cases per year in the United States. A search of the medical literature in 1995 documented approximately 130 cases of scleroderma in women who also happened to have breast enlargement. (This number includes a significant group that was injected with unknown substances such as paraffin or adulterated silicone.)
Since these are rare diseases, they require large-scale epidemiologic studies to determine if a relationship is present. Thirty one such studies from 4 countries, encompassing a cohort of more than 500,000 women, failed to find a statistical relationship between any known or newly recognized disease and silicone. [26, 27, 28, 29, 30, 31, 32, 33, 34, 35]
A panel of experts appointed to review all of the literature for the court with jurisdiction over an implant class action suit summarized their findings as follows:
No association was evident between breast implants and any of the individual connective tissue diseases, all definite connective diseases combined, or the other autoimmune/rheumatic conditions. Sjögren's syndrome was a possible exception to this statement. This entity requires salivary gland biopsy to meet the published diagnostic criteria. Whether biopsy was actually performed for cases in the studies cited is unknown. The remaining criteria based on dryness of the eyes and mouth with possible immunologic alterations are nonspecific and relatively common in any population group. Thus, the accuracy of diagnosis of Sjögren's syndrome in the studies incorporated in this meta-analysis is questionable.
Some claim that silicone causes a totally new syndrome (termed by some silicone-associated disorder), often presenting as a unique variant of fibromyalgia, that may be associated with implants. These claims all have been based on anecdotal reports, and no epidemiologic studies have been performed to support this claim. On October 22, 1995, the American College of Rheumatology issued the following strongly worded statement critical of claims of such new disorders: "The ACR believes that these studies provide compelling evidence that silicone implants expose patients to no demonstrable additional risk for connective tissue or rheumatic disease."
Thus, of the many other symptom complexes, illnesses, and disorders claimed by patients, some physicians, the plaintiffs' bar, and the media to be due to implants, none stand up to scientific scrutiny.
The intensified interest in this issue has generated a great deal of laboratory research in many centers. In some instances, an immune response has been observed in animals, while others have demonstrated positive laboratory findings in a number of women with implants. While animal studies are useful to gain an understanding of processes, extrapolation to humans is often inappropriate. None of the published material to date has presented evidence for a convincing cause-and-effect relationship with any human disease entity. Unfortunately the lay public, the media, and some physicians have equated an immune response, as manifested by laboratory tests, with disease. An immune response is not an immune disease.
As early as 1989, a Plastic Surgery Educational Foundation (PSEF) – sponsored consensus panel on this subject concluded the following:
"There is insufficient information available at this time to determine whether silicone in the form of a breast implant can be implicated as a cause of scleroderma-like syndrome or any other autoimmune disease. Judging from the paucity of reported cases in the very large population of implanted women, if a causal association were to be established, the statistical risk would likely be very low. The presence of risk and magnitude thereof can be determined only by appropriate epidemiological research."
"At present, there is no reason to discourage women from considering breast augmentation on the basis of the risk of acquiring or exacerbating a connective tissue disorder. Until the question is answered by further research, it is wise to inform patients that a theoretical risk might exist, especially if they already have a connective tissue disorder, idiopathic Raynaud's phenomenon, or an affected first-degree relative."
Therefore, if a relationship is present, it must occur in a small number of women who have a genetic predisposition for the disease for which no predictive test is available. The workshop participants repeatedly emphasized that assuming that the presence of an association between 2 findings implies a cause-and-effect relationship is flawed logic. This truism is understood poorly by most and thus is easily is exploited by those who would profit from vilifying the device. In general, while the workshop participants were publicly neutral, a strong air of skepticism privately existed among the participants concerning cause and effect.
In 1991, an FDA panel of experts, having heard all of the then-current evidence that suggested a connection between silicone gel breast implants and rheumatologic disorders, concluded that "the evidence was unconvincing."
At an NIH-sponsored workshop on silicone implants and atypical rheumatic disease, the evidence for a relationship with known typical disease was again acknowledged as not convincing. The participants affirmed that the diagnosis of atypical connective tissue disease was ill-defined, especially for those disorders that rely on subjective symptoms only and are devoid of objective findings or diagnostically specific laboratory findings. The currently classified atypical disorders represent a hodgepodge of subjective symptomatology with or without nonspecific abnormal laboratory tests. They are useful for the purpose of assigning a diagnosis for record keeping, insurance, and patients' needs for labeling but should not be considered as clearly defined entities.
The absence of a clear definition and classification of these vague ailments makes validating their existence impossible. A substantial portion of the rheumatology community is skeptical that such disorders even exist as defined entities. Thus, pinpointing any specific disorder as caused by breast implants is insupportable. Until consensus is reached and these disorders are defined clearly or discredited (estimated to require 10y if started today with adequate funding), abuses and misuse of these diagnoses will continue.
Interestingly, manufacturers and physicians are criticized by the plaintiffs' bar, the media, and unhappy patients for not determining whether implants cause these diseases, when the technology to do so does not exist today, let alone 10-20 years ago.
Finally, several reports state that reversal of the symptomatology can occur with removal of the implants. However, the evidence to date is confusing and suggestive of a placebo effect, the natural ups and downs of the disease, or the results of concomitant treatment.
Silicone Leakage From Intact Implants
The gel in an implant consists of a 3-D, meshlike single molecule that constitutes approximately 20% of the volume. The interstices are filled with a mix of oils of various weights that physically and chemically are bound to the matrix. Only the oil portion bleeds through an intact shell membrane.
Since silicone is hydrophobic, most of the bleed remains adherent to the implant surface. Over time, a few drops are picked up by macrophages, most of which are trapped in the capsule wall. Even smaller quantities can be transported to the lymph nodes.
Infinitesimal amounts of silicone have been found in the breast and surrounding subcutaneous tissue during postmortem examinations of women with implants who died of unrelated trauma. The presence of silicone in these tissues probably resulted from the diffusion of the oil fraction of the implant contents. No silicone was found in the major organs. Animal experiments with radioactive silicone suggest that some immeasurable amount may escape beyond this into all body tissues, but this has never been documented in humans. Presumably, if it occurs, the quantities are too small to measure.
Because silicone is hydrophobic, it is unlikely to be transported by any mechanism other than macrophage migration or local diffusion.
As noted, various silicones in minute quantities already exist in all humans. No evidence suggests that the gel contents have any different metabolic effect on the body than the solid silicone envelope or any other benign foreign body. Thus, little concern is necessary about the small amount of silicone that passes through the implant shell.
Implant Rupture and Leakage
The true failure incidence of gel-filled implants remains elusive for 2 reasons. First, the incidence of silent rupture is difficult to determine. Second, current reports in the literature are contaminated by selection bias, since they report on women who seek care for a perceived problem. Additionally, more recent publications do not separate their findings by device generation or manufacturer. [36]
A retrospective study by Hadad et al of 284 women (542 breasts) indicated that in breast augmentation mammoplasty with silicone gel implants, larger implant size and use of the submuscular, as opposed to the subglandular, plane increase the risk of implant rupture. The average implant age at explantation (among the study’s patients, who were undergoing implant exchange) was 10 years, with the investigators finding the relative proportion of submuscular to subglandular ruptured implants to be 64%. [37]
If an implant shell is ruptured, the escaping gel usually is contained in the surgical pocket by the scar envelope. No evidence indicates that this constitutes any kind of health hazard or has any different physiologic effect than the intact implant. (Broken inflatable devices release only harmless saline, which is absorbed promptly by the body, resulting in rapid deflation.)
At one time, the belief was held that most ruptures resulted from the then-common practice of closed capsular rupture (a technique, discussed later in the article, for treating firm, contracted capsules), but this is hard to document. Thus, the expected capsule tear from closed capsular rupture may mask implant disruption. For some patients, however, the tear may be silent or cause so little sensation as to be ignored. Prompt consultation with a plastic surgeon is recommended if rupture is suspected.
One dilemma facing the clinician is what to advise patients with asymptomatic intracapsular rupture. With the currently available evidence of gel safety, providing a medical justification for explantation is difficult unless extravasation occurs into the breast tissue, interfering with mammography or presenting as a lump.
A study by Seigle-Murandi et al looking primarily at Allergan silicone implants indicated the estimated risk for rupture-associated removal to be 0.5% at 1000 days, 6% at 2000 days, and 14% at 3000 days. However, these figures were found to vary according to the specific type of Allergan implant; one of the types of round, macrotextured implants had a risk for rupture-associated removal of 33% at 3000 days, while the anatomically shaped range of implants had a 6% risk over the same time period. [38]
Leakage beyond the scar envelope
Occasionally, when the scar envelope is torn and the shell of the implant is disrupted, the gel can be driven into the local tissue planes or adjacent breast tissue. As in all benign tumors of the breast, the tissue containing the extravasated silicone should be removed, since it may mask or mimic a breast cancer. On even rarer occasions, silicone gel has been known to extend fairly far along the tissue planes of the chest wall, down into the abdomen, into the arm, or directly into the breast tissue.
Isolated reports have described silicone extending into the nerve sheaths of the brachial plexus and peripheral nerves, with resultant signs of compressive neuropathy, or extending into the skin, resulting in induration, necrosis, and deformity. These are extremely unusual occurrences and generally are observed in women who received low-viscosity gel, thin-walled shell implants, which were used for a short time in the late 1970s, or who had associated injections of silicone or other materials.
Within 2-6 weeks, extravasated gel is trapped in a new scar capsule. The larger droplets then are invaded by strands and septa of connective tissue, ultimately subdividing the material into smaller and smaller microdroplets. This results in a granuloma, which may be firm enough to palpate. Most silicone-invaded tissues probably remain soft until an inflammatory process produces enough edema to make them palpable.
Extravasation beyond the surgical pocket can produce some unpleasant physical deformities, lumps, and masses. No health reason exists to radically excise this material, except from the breast tissue (if removal would cause deformity) and possibly from nerve sheaths if they are symptomatic. Following removal of a sufficient amount of gel to minimize or eliminate a deformity, a patient may have to accept the residual firm plaques of silicone as permanent.
Fortunately, major rupture with distant extravasation is extremely rare and usually is easy to diagnose. Most women become aware of a torn capsule from the associated pain or discomfort, as well as from the physical presence of a new mass.
Rupture diagnosis
Making the diagnosis of an intracapsular break without direct exploration may be extremely difficult. Mammography is rarely useful. Preliminary studies indicate that ultrasonography may be helpful, and a phase 4 prospective study is being conducted to determine the sensitivity and specificity of high-resolution ultrasonography in evaluating current implant designs. [39] (For more information, see Imaging in Breast Implant Rupture.)
Magnetic resonance imaging (MRI) using a dedicated breast coil is the best diagnostic tool; in experienced hands, its success rate is as high as 85%. Physical examination alone occasionally can provide a clue to the possible rupture of a broken device. Suspect rupture if a change in the character of the device is present, such as new, persistent, burning discomfort on one side or a change in the softness, texture, or shape of the implant.
Pregnancy and Lactation
Using silicon levels as an indicator of silicone content, current studies show no silicone increase in the human milk of implanted women. A small sampling of milk measured directly for silicone also has failed to detect levels any higher than those in the controls. Even if milk did contain silicone, the amount would be far less than an infant would receive from a dropperful of pediatric Mylicon, in which silicone antifoam is the main ingredient. (This medication is available without prescription to treat infant gas with FDA approval.)
Some have been concerned that mother's milk from a woman with a polyurethane device may contain TDA. The only evidence for this came from a sample in which TDA was measured in parts per billion, a level the investigator himself agreed was below the reliability threshold of the test.
Lactation difficulties
New evidence suggests that women with implants may be more likely to have difficulty with lactation than those without. A report involving 42 cosmetically augmented women showed an increased incidence of inadequate milk supply over controls. [40] This was especially true among women with periareolar insertions. (The same has been found to be the case for women who have undergone periareolar incisions for nonimplant surgery.)
The evidence suggests that lactation insufficiency is related either to congenital glandular inadequacy, usually associated with smaller breasts, or with periareolar access surgery that damages the ductal system. [41, 42, 43] This suggests that the periareolar approach should be directed subcutaneously around the lower pole of the breast to gain entry to the retroglandular space rather than directly through the breast tissue, to retain the woman's prospects for nursing. [44, 45]
Closed Capsular Rupture
For women who develop firm, contracted capsules, a technique termed closed capsular rupture can, on some occasions, result in instant relief. [46] This involves forceful squeezing of the breast, which disrupts the scar envelope and enlarges the pocket, thus restoring softness. This has been an attractive procedure, for when it works it is simple, only momentarily painful, and instantaneous.
The tear strength of the scar envelope varies significantly among women. Some envelopes tear very easily (and often painlessly), but in other cases the scar is so tough that it cannot be torn without applying excessive force. In still other cases, a partial tear can occur that can lead to a small hernia of the implant, which may result in an unsightly or radiologically confusing diverticulum. The stretching of the device into the diverticulum can produce a stress concentration or can strain the shell beyond its elastic limits, thus resulting in rupture.
Although the recurrence rate following closed capsular rupture is high, some women find this process so easy that they have their husband or others perform it on them as needed. Unfortunately, in a patient with a broken implant, a tear in the capsule and the force used in this technique can drive the loose gel into adjacent tissues.
Closed capsular rupture is not recommended by the implant manufacturers and voids any warranty. However, it is an attractive solution to the problem of firmness when used judiciously and when the woman is appropriately informed. The only other alternatives are surgical scoring or excision of the tight capsule. [46] All of these techniques may result in recurrence.
Lab Test Considerations
No specific laboratory tests provide useful information concerning the body content of silicone or 2, 4, toluene diamine (TDA) or the systemic consequences. Some entrepreneurial laboratories are soliciting specimens of blood, urine, or mother's milk to analyze for silicon, platinum (contained in the catalyst used to polymerize or cure the gel), and TDA or for immunologic panels supposedly useful in diagnosing "silicone-related diseases."
Silicon
Elemental silicon is essential to many body tissues and is normally present in blood, milk, connective tissue, and most organs. Its concentration varies widely with diet, geography, the local water supply, and the patient's beer consumption. However, the silicon content of blood, milk, or tissue bears no known relationship to the silicone content in the body, with the exception of specific tissues such as those of the breast capsule and the breast's immediate environs.
TDA
TDA measurements represent a specific testing problem, since the commonly used methodology appears to produce TDA from the test process. As early as 1992, a letter to Congresswoman Lloyd from the FDA included the following:
"There is no current information in the medical literature regarding the clinical utility of any test or set of tests for monitoring or diagnosing disease specifically in patients with silicone breast implants. Use of procedures described under these conditions would be classified as investigational by the FDA."
Later, a position paper from the College of American Pathologists stated the following in its opening paragraph:
"It is the position of the College of American Pathologists that laboratory tests measuring blood, urine, or tissue silicon, silicone, toluene diamines, or related substances are not currently indicated or useful for purposes of medical management of individual breast implant recipients.
Serum auto-antibody tests or panels are useful in the evaluation of individual patients suspected of having collagen vascular disease, whether or not they have received breast implants, but such tests provide no findings uniquely indicative or supportive of purported silicone induced autoimmune disease in implant recipients. Interpretation of such panels as 'consistent with silicone reaction' for example are not supported by the medical literature."
Both of these statements remain valid as of this date. In 1994, the Centers for Disease Control and Prevention (CDC), which has authority over clinical laboratories, issued a similar statement. As in any disorder, laboratory testing should be specific for the symptom or disease. Any conclusions concerning future risks or current illness from silicone or polyurethane drawn from clinical laboratory results are spurious. Discourage patients from requesting unnecessary tests that are meaningless and may serve only to confuse or reinforce anxiety. A literature review relating to breast implant litigation is available here.
Regulatory History and FDA Action
At hearings held in October 1991, an advisory panel to the FDA concluded that "there was no evidence that [silicone gel – filled breast] implants are unsafe but that there was insufficient evidence to prove safety." The panel also concluded that the devices were valuable enough for either cosmetic or reconstructive patients to recommend that the implants be left available for all who wish them under the public health necessity provision of the law.
Despite this recommendation, FDA Commissioner David Kessler announced a temporary and voluntary moratorium on the use of gel-filled implants on January 6, 1992. Commissioner Kessler recommended that women who already had the device and were trouble free need not have them removed, but that no women should have them newly inserted.
A panel convened in February changed the previous panel's recommendations. The new panel advised stricter controls on silicone gel implants for cosmetic purposes but full access for reconstruction under a structured "adjunct" protocol with institutional review board (IRB) approval. All women receiving implants were to be encouraged to participate in a registry, and strict study protocols were to be instituted.
On April 16, 1992, Commissioner Kessler confirmed the panel's recommendations. Until the protocols were in place, limited access to the gel-filled implant was permitted under the "urgent need" provision for women whose reconstruction is in progress, for replacement of broken devices, for immediate breast reconstruction, and for other specific cases of clinically appropriate need. The implants were not approved for new cosmetic augmentations except under a limited experimental protocol.
In September 1992, the FDA approved the "adjunct" protocol permitting use of the gel implant for all reconstructive purposes under a limited study program monitored by an IRB. More detailed "core studies" were to be instituted for a limited number of patients and investigators. These would include separate studies for cosmetic and reconstructive patients.
In 2000, the FDA wrote to implant manufacturers advising them of their requirements for additional data and encouraging them to begin a final 18-month study leading to submission of a premarket approval (PMA). In 2006, these studies were completed, and the FDA approved silicone gel implants for all breast enhancement purposes; however, the FDA has required continued tracking of patients.
Impact on Health Insurance
The entire breast implant controversy continues to impact adversely on some patients' insurability, since a number of carriers have excluded coverage for any breast disease if the woman has implants. Fortunately, due to the efforts of the American Society of Plastic and Reconstructive Surgeons (ASPRS), this practice seems to have diminished, but isolated reports of imposition of these riders still surface. Inappropriate association of other disorders to these devices may further compromise coverage. It behooves plastic surgeons, as advocates for patients, to intervene on their behalf and act to obtain recession of inappropriate exclusions.
-
The molecular structure of silicone.
Tables
What would you like to print?
- Overview
- History of Breast Implants
- Benefits
- Contraindications
- Properties and Uses of Silicone
- Repeat Surgery
- Capsular Contracture
- Carcinogenesis
- Polyurethane-Coated Implants and Cancer
- Breast Cancer Detection Concerns
- Rheumatologic Disorders
- Silicone Leakage From Intact Implants
- Implant Rupture and Leakage
- Pregnancy and Lactation
- Closed Capsular Rupture
- Lab Test Considerations
- Regulatory History and FDA Action
- Impact on Health Insurance
- Show All
- Tables
- References