Overview
When a patient with rheumatoid arthritis (RA) develops joint deformities in the hand or wrist that are unresponsive to medical management, surgical intervention is often necessary. [1, 2, 3] These deformities lead to loss of the ability to grip, grasp, and pinch, often leaving the patient unable to perform the activities of daily living. See the images below.
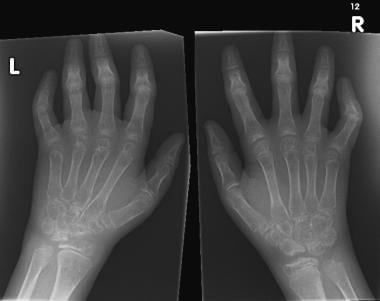 Widespread osteopenia, carpal crowding (due to cartilage loss), and several erosions affecting the carpal bones and metacarpal heads in particular in a child with advanced juvenile rheumatoid arthritis (also known as juvenile idiopathic arthritis).
Widespread osteopenia, carpal crowding (due to cartilage loss), and several erosions affecting the carpal bones and metacarpal heads in particular in a child with advanced juvenile rheumatoid arthritis (also known as juvenile idiopathic arthritis).
Appropriately timed surgical intervention helps alleviate pain, improve function, retard progression of the disease, and improve appearance. [4] Consequently, independence is greater and self-image is improved.
A study by Okura et al reported that out of 67 patients who underwent primary elective elbow, wrist, or hand surgery for rheumatoid arthritis (RA), almost 85% expressed satisfaction with the treatment’s outcome at the involved site, particularly with regard to pain relief, at 10-year follow-up. [5]
The surgical treatments for RA of the hand and wrist include synovectomy, tenosynovectomy, tendon realignment, reconstructive surgery or arthroplasty, and arthrodesis. [6] Surgical treatment is much more likely to be successful if it is implemented early in the course of the deformity. If the patient does not receive timely referral to a hand surgeon, the resultant function of the hand may be severely compromised.
A study by Ishikawa et al on the systemic effects of rheumatoid arthritis (RA)–related orthopedic surgery found significant improvement in physical function and reduction in disease activity, at 6 and 12 months postoperatively. Of the study’s 276 patients, 74 (26.8%) underwent surgery to the wrist, and 63 (22.8%) underwent surgery to the hand. [7]
Globally, the number of surgeries for RA has reportedly been trending downward, possibly as a result of improved medical therapies for the disease. A retrospective, single-center study by Tominaga et al found that over a 20-year period, there was a 50.3% decrease in the total half-year period prevalence proportion (HPP) of RA-related surgeries, with significant reductions in knee, hip, shoulder/elbow, and hand procedures. Hand procedures declined by 41.8%. There was, however, a significant increase in the volume of foot/ankle joint surgeries over this period. [8]
Tenosynovitis
Tendon sheaths are lined by paratenon and synovium; therefore, the tendons are affected by the same disease process as joints. In fact, symptoms of tenosynovitis may occur before those of intra-articular disease. The affected sites are (1) the dorsal and volar aspects of the wrist, because the tendons are covered by synovium as they pass under the flexor and extensor retinaculum and under the wrist, and (2) the volar aspect of the digits, because the tendons are covered by synovium in the fibro-osseous canals in the finger. [4] Synovitis of the tendons can cause pain, dysfunction, and eventual rupture of tendons.
Early in the process, synovial proliferation results in the accumulation of fluid that causes swelling. As the tissue continues to proliferate, the synovium thickens. Furthermore, small fibrinoid rice bodies can develop in the tendon sheath. Hypertrophic synovial tissue begins to invade and weaken the tendon, eventually leading to rupture. Tendon rupture may also be a result of attrition of the tendon from bony spicules and osteophytes. Rheumatoid nodules can also develop within the tendons and within the subcutaneous tissue.
Dorsal tenosynovitis in the wrist is usually detected when the dorsum of the wrist becomes swollen. Minimal pain may be present, but bogginess and crepitus can be appreciated with palpation. This swelling may even be the first sign of RA and may involve any combination of extensor tendons. In early cases, spontaneous remission can occur. Furthermore, rest, steroid injections, or medical management may induce remission. As dorsal tenosynovitis becomes more apparent, the likelihood of tendon rupture increases. Tenosynovectomy is usually recommended if symptoms do not improve after 4-6 months of medical therapy. After a tenosynovectomy, tendon rupture rarely occurs and complications are infrequent, yet postoperative adhesions may occur. Tendon adhesions result in an extensor lag of metacarpophalangeal (MP) joints or decreased active finger flexion.
Swelling is less obvious in wrist flexor tenosynovitis because of the thicker skin on the volar aspect of the hand. Decreased passive and active motion of the fingers is caused by impairment of the free-gliding movement of the flexor tendons through the sheaths as the thickened synovium proliferates. Proliferative synovitis in flexor tendon sheaths can cause compression of the median nerve and can produce the symptoms of carpal tunnel syndrome. Tendons can also adhere to each other and may rupture. To avoid the consequences of prolonged median nerve compression, early carpal tunnel release and tenosynovectomy is recommended in patients in whom conservative means have failed.
A study by Matthijssen et al indicated that magnetic resonance imaging (MRI)–detectable tenosynovitis is highly sensitive (85%) for RA, with the sensitivity for anti-citrullinated protein antibody (ACPA)–positive RA being 88%, and for ACPA-negative RA, 82%. MRI-detectable tenosynovitis showed a significantly greater sensitivity for RA than for psoriatic arthritis, spondyloarthritis, reactive arthritis, and self-limiting undifferentiated arthritis. [9]
Flexor Tenosynovitis in the Fingers
Synovial proliferation produces discrete rheumatoid nodules on tendons, which can result in trigger finger. The size and location of these nodules on the flexor tendon determine the degree of triggering.
Four types of trigger finger occur in RA. Type 1 is similar to nonrheumatoid stenosing tenosynovitis, in which the tendons catch at the first annular pulley during flexion secondary to small, localized hyperproliferation of the synovium. In type 2, the nodules form in the distal palm and cause the finger to lock in flexion. In type 3, nodules on the flexor digitorum profundus (FDP) tendon near the second annular pulley (over the proximal phalanx) lock the finger in extension. Type 4 trigger finger results from generalized tenosynovitis within the fibro-osseous canal. Active motion is more restricted than passive motion, and contracture and stiffness result.
Flexor tenosynovectomy and nodule excision are recommended for all types of tenosynovitis and trigger finger.
Tendon Rupture
The 2 main causes of tendon rupture are chronic attrition of the tendon over a bony prominence and synovial invasion of the tendon. The most frequent sites for extensor tendon rupture are at the distal end of the ulna or at the Lister tubercle, which acts like a fulcrum for the extensor pollicis longus (EPL) tendon.
Sudden loss of finger extension or flexion is the cardinal sign of tendon rupture. Usually, it is painless and can occur during normal use of the hand. Patients with RA frequently ignore the new development, because it may be subtle in the context of the other constraints on their normal function.
Extensor tendon ruptures
Commonly affected extensor tendons are the EPL, extensor digiti quinti, and extensor digiti communis. Multiple extensor tendon ruptures are usually initiated by attrition of a single tendon and progress in a radial manner, frequently beginning with the small finger extensor.
A retrospective study by Hsueh et al indicated that risk factors for spontaneous extensor tendon rupture in patients with RA of the wrist include RA of over 8 years’ duration, persistent tenosynovitis lasting for more than 1 year, and a Larsen score of over 4. [10]
EPL tendon rupture may not significantly affect the thumb interphalangeal (IP) joint extension, because this motion is controlled by the intrinsic muscles and by the EPL. In contrast, extension at the thumb MP joint is usually reduced, because the extensor pollicis brevis cannot compensate for deficient EPL function. To test for EPL tendon rupture, palpate the tendon at the wrist while the patient extends the thumb with the palm resting on a flat surface. Active testing may also reveal some weakness and pain at the site of rupture.
Significant deformity or functional loss is an indication for repair by end-to-end repair, tendon graft, or tendon transfer to avoid flexion contraction. [4, 11] Tendon transfer with the extensor indicis proprius or extensor carpi radialis longus is the preferred method. [4] No deficit in independent index finger motion or wrist extension results using these tendons. Furthermore, this transfer avoids the complication of adhesion formation that can occur with tendon grafts. End-to-end repair is usually not possible because of the delay that frequently occurs between the time of the injury and the time the patient presents for treatment.
Extensor tendon rupture due to RA also frequently affects the small finger, causing extensor lag at the MP joint. Loss of extensor digiti quinti function reduces small finger extension at the MP joint by 30-40°. Any greater loss usually indicates the extensor digiti communis tendon has also been affected. The Vaughn-Jackson lesion is a sign of loss of extension in the little and ring fingers due to rupture of these tendons at the distal ulnar head, which can result from caput ulna syndrome.
Small finger extension can be tested by having the patient extend the small finger while the index, middle, and ring fingers are held in flexion. A small finger extensor tendon rupture from RA should be repaired early, because this defect generally leads to multiple tendon ruptures. Repair is usually accomplished by attaching the distal tendon end to an adjacent extensor tendon. [4] Dorsal tenosynovectomy and removal of bony spicules that may have worn down the tendon are also required to eliminate the ultimate cause.
Sequelae of RA that mimic extensor tendon ruptures
When diagnosing extensor tendon ruptures secondary to RA, 3 other sequelae of RA that mimic this problem should be considered. First, MP joint dislocation produces a fixed deformity with a flexed and ulnarly deviated finger. Second, volar and ulnar subluxation of the extensor tendons into the gutters between the phalanges limits extensor function as the extensors essentially become flexors in this position. In this condition, the patient can sometimes maintain MP extension actively after the finger is passively extended. Finally, paralysis due to posterior interosseous nerve compression can also cause symptoms similar to extensor tendon rupture when it affects the common extensor muscle. Differentiation between tendon rupture and paralysis can be difficult, and the most reliable diagnostic maneuver is to test for the presence of MP joint extension while flexing the wrist (tenodesis effect). Because the tendon is not in continuity when it is ruptured, wrist flexion does not restore MP joint extension.
Flexor tendon ruptures
The diagnosis of flexor tendon rupture is more straightforward. The flexor pollicis longus is the most common flexor tendon to be affected by RA. Patients with this condition are not able to actively flex the thumb IP joint. The diagnosis can be slightly complicated if chronic stiffness or hyperextension is present in this joint. Similarly, loss of function in the proximal IP (PIP) joint or the distal IP (DIP) joint suggests rupture of the flexor digitorum superficialis (FDS) or profundus (FDP), respectively. Rheumatoid nodules can limit FDP function and produce symptoms similar to FDP tendon rupture; therefore, this diagnosis should be considered. A palpable nodule can aid in differentiating between these conditions. Furthermore, when the flexor tendon is ruptured, the affected finger also has a more extended posture in the resting position.
To make the diagnosis of concomitant FDP and FDS tendon rupture, passive motion of the finger must be present in the absence of active motion at the DIP and PIP joints. The flexor tendons can rupture at multiple sites in the hand, including the wrist, palm, and fingers. Surgical exploration is usually required to determine the exact location of the rupture.
Treatment of flexor tendon rupture varies with the degree of functional disability. Rupture of the FDP may not require repair if the DIP joint remains stable, despite compromised active flexion. The treatment of a rupture of the FDP tendon is also complicated because, through restoration of the FDP tendon, the FDS tendon or PIP joint may become compromised, resulting in a stiff and functionless finger. The treatment of most FDP ruptures is best served with a tendon or arthrodesis at the DIP joint. FDS tendon rupture alone usually does not require repair, because the functional loss is minimal. Rupture of both the FDP and FDS tendons does require surgical intervention, because the functional loss is obvious.
In the wrist and palm, the ruptured tendon can be sutured to an adjacent tendon, but within the fibro-osseous canal in the finger, this is not an option. Primary repair is seldom an option because of the significant restriction of the tendon within the canal. Also, tendon grafts have traditionally yielded poor results. [4] Staged tendon reconstruction can usually be effective, but this technique is also fraught with poor results. [4] A last-resort treatment for older patients with severe disease may be fusion of the PIP and DIP joints in a functional position.
The Metacarpophalangeal Joint in RA
The condylar structure of the MP joint, which permits motion in 2 planes, makes the MP joint inherently more unstable than the IP joints; therefore, the distorting effects of RA are more pronounced. The classic deformities associated with RA of the MP joints are ulnar drift, which is made up of ulnar shift and ulnar deviation, and volar dislocation. The cumulative effects of various factors contribute to these deformities. Initially, the MP capsule and ligamentous structures are stretched by the proliferation of the synovium, which loosens the collateral ligaments and decreases joint stability. Normally, in the flexed position, minimal lateral movement occurs at the MP joint, but, with increased laxity of the collateral ligaments, up to 45° of lateral deviation occurs in this position. [4]
Ulnar drift
With loss of MP stability, other forces on the MP produce the characteristic ulnar drift. For example, wrist collapse contributes to ulnar drift. Weakened radiocarpal ligaments cause radial rotation of the metacarpals and carpus on the radius, which results in ulnar deviation of the MP joint via the Z mechanism. This phenomenon describes the consequence of an imbalance of opposing forces at a joint; when a particular joint is continuously angulated in one direction, the joints on either side of it will adopt the opposite position if no physical impediments are present. [4] Moreover, the extensor tendons can be shifted or dislocated in the ulnar direction and contribute to the ulnar drift. The normal ulnar shift of the fourth and fifth extensor tendons during flexion of the MP joints is exaggerated when the radial side of the transverse lamina fibers stretches as a result of synovial proliferation.
Volar dislocation
Volar dislocation or subluxation is also caused by weakening of the collateral ligaments and by weakening of the dorsal extensor mechanism when the extensor tendons are dislocated between the metacarpal heads. As a result, no force counters the extrinsic and intrinsic flexors, and a flexion contraction at the MP joint occurs, which is evident by prominent metacarpal heads.
Treatment
Conservative treatment is advised for RA of the MP joints to see if control can be gained with medication therapy alone. [4] Recurrence is always a possibility postsynovectomy, and approximately 30-50% of patients may undergo spontaneous remission. Synovectomy is indicated in the RA patient whose MP joint RA is refractory to 6-9 months of medical treatment and has persistent MP joint synovitis with minimal joint deformity and minimal radiographic evidence of RA. Additionally, extensor tendon relocation with synovectomy is indicated for an RA patient who also has early volar subluxation and ulnar drift, especially if the patient is young and the disease is not rapidly progressing. Subluxed extensor tendons fall into the ulnar gutters of the MP joint. The patient may not be able to extend this finger once it is in the flexed position. However, the finger can maintain extension if passively placed into this position.
The arthroplasties of the MP joint can incorporate reconstruction of the soft tissues only or involve complete joint replacement. Soft-tissue arthroplasty usually incorporates an element of synovectomy with MP joint stabilization, such as reconstruction of the radial collateral ligament or tendon realignment. Joint replacement is indicated for MP joint deformity or subluxation when pain is not controlled or function is impaired. Various MP joint implants are available for joint replacement. [12]
The MP joint replacement procedure involves making either a transverse incision across the entire dorsum of the hand at the MP level or individual longitudinal incisions over each MP joint. Less chance of interference with the lymphatics and venous outflow occurs with the individual longitudinal incisions, but the transverse incision is most commonly used for ease of access. The dissection is carried down to the paratenon level. An incision is made on the ulnar aspect of the extensor tendon, and the tendon is reflected radially. Care is taken to not breach the integrity of the capsule at this time. An incision is made longitudinally in the capsule of the MP joint. Using a periosteal elevator, the metacarpal head is freed of any soft-tissue attachments to the metaphyseal flare.
Using an oscillating saw, a true cut is made, maintaining 90° in line with a long access of the metacarpal and in the coronal plane. The metacarpal head is then discarded. An electrical burr or a Christmas-tree type of rasp can be used to ream the medullary canal. The medullary canal of the proximal phalanx is then identified through a burr hole on the articular surface of the base of the phalanx. Osteophytes are removed from the proximal phalanx at this time with a rongeur. The intramedullary canal of the proximal phalanx is reamed. Care is taken so that the rectangular opening for both the metacarpal and the proximal phalanx are square in line with the axial direction of both the metacarpal and phalanx. Appropriate sizes are then placed inside the medullary canals.
Using a no-touch technique, the definitive prosthesis is then introduced. The capsule is repaired, and the extensor tendon is subsequently aligned. Some authors prefer to reconstruct the collateral ligaments at this time. Care is taken at the initial dissection to preserve as much of the collateral ligament as possible. The skin is then closed using nylon suture, and a splint is applied with the fingers in extension and in a neutral position.
Approximately 4-5 days after the procedure, the patient is fitted for a dynamic outrigger splint that maintains extension in an appropriate anatomic position of the fingers while the patient undergoes active flexion exercises. Night splints are manufactured to maintain the fingers in extension. Splinting is required for the next 4-8 weeks. Follow-up radiographs are obtained to confirm the appropriate positioning of the implants.
A study by Funamura et al reported that at average 11.4-year follow-up, MP joint arthroplasty using the Swanson implant was providing effective pain relief and deformity correction but had proven somewhat problematic with regard to implant durability and mobility. Of MP joints that underwent surgery, the number of tender joints was reduced from 24 (27.6%) to 1 (1.1%), and the number of swollen joints decreased from 28 (32.2%) to 2 (2.3%). Eight joints (9.2%), however, were found to have implant fracture, while the average active range of extension/flexion shrank from -46.3°/65.9° to -32.3°/56.6°. Grip and pinch strength were not known to have significantly changed, but patients were satisfied with the operative results on pain and hand appearance. [13]
Crossed intrinsic transfer of the extensor tendons from the ulnar side to the radial side of the adjacent finger to increase stability is another surgical option. Intrinsic release to alleviate intrinsic tightness eliminates the dorsal digital expansion tightness that is one of the subluxing forces on the MP joint.
Finally, MP joint arthroplasties can provide long-term relief. A patient with good hand function in the absence of pain is not a candidate for arthroplasty, even if obvious MP joint deformity is present, because surgery may not improve the hand function and could decrease grip strength.
The PIP and DIP Joints in RA
The swan-neck and boutonniere deformities are common in, but not unique to, patients with RA. In the swan-neck deformity, the DIP joint is flexed and the PIP joint is hyperextended. The boutonniere deformity is characterized by the opposite, with DIP joint hyperextension and PIP joint flexion. Both are caused by an imbalance of the forces on these joints due to synovial proliferation.
Swan-neck deformity
Swan-neck deformity occurs in approximately 50% of patients with RA; it may also be congenital or traumatic in nature. [14]
Surgical intervention for the swan-neck deformity is advised when active flexion of the PIP joint from its hyperextended position is not possible or occurs with a bothersome snap. Multiple surgical procedures are available for the correction of this deformity, and accurate staging of the deformity is necessary for selection of the most appropriate surgical technique. The staging is based on the condition of the articular cartilage—which is determined by radiography—and on the flexibility of the PIP joint.
When evaluating the finger, the proliferation of synovium around a joint can be detected by observing fluctuant swelling beneath the examiner's fingers when the joint is held in 45° of flexion.
The active and passive ranges of motion of each joint should be measured with a goniometer. Hyperextension is recorded as a negative value. In addition, the lateral stability of each joint should be tested by applying 3-point pressure, with the finger in extension.
The finger in question should also be tested for intrinsic (interosseous and lumbrical) muscle tightness. The examiner should hold the MP joint in full, passive extension and flexion, and then gently flex the PIP joint with the other hand. In the normal finger, full PIP joint flexion is possible in extension and flexion of the MP joint. In contrast, in the presence of intrinsic tightness, resistance to PIP joint flexion is encountered when the MP joint is in extension (and the intrinsics are already passively stretched), although when the MP joint is in flexion, passive PIP flexion is possible. The angle of passive PIP flexion is determined with a goniometer and recorded.
Classification of swan-neck deformities
Nalebuff classified swan-neck deformities into the following 4 types, on the basis of PIP joint mobility. [14] The classification is widely accepted and aids in the choice of surgical treatment for this complex condition.
-
Type I - PIP joints are flexible in all positions.
-
Type II - PIP joint flexion is limited in certain positions.
-
Type III - PIP joint flexion is limited in all positions.
-
Type IV - PIP joints are stiff and have a poor radiographic appearance.
An alternative classification was proposed by Welsh and Hastings, who classified swan-neck deformity as mobile, snapping, or fixed, on the basis of the condition of the digital intrinsic muscles [15] and subdivided swan-neck deformity into 2 types:
-
Type I - Caused primarily by PIP joint disease
-
Type II - Caused primarily by MP joint disease
Nalebuff type I deformity
Swan-neck deformity can arise at the PIP or DIP joint; in either case, it can lead to the classic appearance of PIP joint hyperextension with DIP joint flexion. Patients with type I deformity maintain the ability to actively flex the PIP joint. When the deformity originates at the PIP joint, it is caused by stretching of the capsule secondary to active synovitis or rupture of the flexor digitorum superficialis tendon, removing the restraint to PIP joint hyperextension.
If the synovitis involves the DIP joint, the deformity begins with stretching or rupture of the terminal tendon attachment of the extensor mechanism to the distal phalanx, resulting in a mallet deformity. (See also the article Mallet Finger.) This subsequently causes extensor mechanism imbalance, with relative overpull of the central slip; these problems, together with laxity of the PIP joint's volar plate, result in PIP joint hyperextension.
The treatment of type I deformity is focused on correcting PIP joint hyperextension and restoring DIP joint extension. Conservative treatment can be used, with Silver Ring splints (Silver Ring Splint Co., Charlottesville, Va) employed to permit active PIP flexion and limit hyperextension of the PIP joint. Alternatively, an inexpensive figure-8 thermoplastic splint can be fashioned by a hand therapist. These splints can be useful in the early stages of the disease. [16]
Van der Giesen et al found that silver ring splints and commercial prefabricated thermoplastic splints were equally effective in treating swan neck deformities in patients with RA. [17] In a randomized crossover trial, 47 patients used both splints for 4 weeks, with a washout period of 2 weeks, and then used the preferred splint for another 12 weeks. Improvement in dexterity, as measured with the Sequential Occupational Dexterity Assessment (SODA) score, was similar with the 2 splints. Patients using the silver ring splint had SODA score improvement of 11.2; those using thermoplastic splints had improvement of 10.8. The only difference in overall clinical outcome or patient satisfaction was a significantly higher score in 3 items of satisfaction in the silver-ring-splint group. [17]
If splints are not tolerated, several procedures can be considered, including DIP joint fusion (soft-tissue procedures at the DIP joint are unsuccessful) and PIP joint flexor tenodesis, in which a volar zigzag incision is made over the PIP joint to expose the flexor tendon sheath; the sheath is opened proximally to the A1 pulley, and the flexor digitorum superficialis is separated from the sheath, creating a slight flexion contracture of the PIP joint. Another option is retinacular ligament reconstruction and dermodesis, in which an elliptic wedge of skin is removed from the volar aspect of the PIP joint, and the skin defect is closed with the digit in flexion. However, this procedure is usually only of temporary value, because the skin stretches out with time.
Nalebuff type II deformity
A type II deformity has an appearance similar to that of the type I deformity; however, PIP joint flexion is influenced by the position of the MP joints. When the MP joints are extended or radially deviated, passive PIP joint flexion is limited; when the MP joints are flexed or ulnarly deviated, a greater degree of PIP joint flexion is possible. As the patient's RA disease continues to progress, radial deviation of the metacarpals and volar subluxation of the MP joints increase secondary to increased tightness of the intrinsic muscles. Consequently, a swan-neck deformity develops.
The treatment of a type II deformity centers on the relief of intrinsic tightness, which is accomplished using intrinsic release. In this procedure, a dorsal longitudinal incision is made over the proximal phalanx, exposing the extensor mechanism. A rhomboid portion of the ulnar extensor aponeurosis is then resected (radial as well, if the tightness is severe). The surgeon resects the lateral band(s) through which the abnormally tight intrinsics have produced MP flexion and PIP hyperextension. In patients with severe involvement of the MP joints, silicone-implant arthroplasty is performed, combined with the rebalancing of the intrinsics and the long extensor tendons. [18, 19]
Nalebuff type III deformity
A type III deformity is characterized by a significant reduction of PIP joint motion, but with well-preserved joint spaces, as depicted on radiographs. The stiffness is caused by contracture of the extensor mechanism, collateral ligaments, and skin. The initial goal of the surgical reconstruction of a type III deformity is the restoration of passive motion to the PIP joint. This may be accomplished by using 1 or more procedures, including PIP joint manipulation, lateral band mobilization, and flexor tenosynovectomy or tenolysis.
PIP joint manipulation involves dorsal skin release distal to the PIP joint to allow the skin edges to spread and scar contraction to occur in 2-3 weeks. This leads to a linear scar.
In lateral band mobilization, the lateral bands are freed from the central slip mechanism and the joint is gently manipulated into full flexion without releasing the collateral ligaments or lengthening the central slip. However, De Bruin et al reported disappointing results in their evaluation of the long-term effect of lateral band translocation for swan-neck deformity in 62 fingers of patients with cerebral palsy. [20] Although correction was successful in 84% of the fingers at 1 year after surgery, after 5 years, the success rate had declined to 60%. The authors additionally noted that they found no relationship between concomitant surgical procedures and swan-neck recurrences and concluded that lateral band translocation should not be considered a long-lasting procedure in these patients. [20]
Flexor tenosynovectomy or tenolysis involves exposing and applying traction to the flexor tendons of the distal palm. [21] (See also the article Hand, Flexor Tendon Lacerations.)
Once passive motion has been restored, the deformity may be corrected with the previously mentioned procedures. Postoperative splinting and exercises are implemented by a hand therapist, under the supervision of the surgeon, to maintain the gains that were achieved surgically.
Nalebuff type IV deformity
Patients with a type IV deformity have stiff PIP joints and associated radiographic changes consistent with advanced intra-articular disease. These deformities require a salvage-type procedure—namely, arthrodesis or arthroplasty. In deciding which of these procedures to perform, it is important to consider the status of adjacent joints, flexor tendons, and ligaments. It is also important to assess the function of the adjacent fingers. Fusion is particularly useful for the index and middle fingers, because these digits need lateral stability when opposed to the thumb during pinch. Arthroplasty is recommended for the ring and small fingers, where mobility aids grasp. If the MP joints require arthroplasty, PIP joint fusion is recommended, although it has been suggested that arthroplasty can be performed.
Proximal joint fusion involves a curved dorsal skin incision. A longitudinal incision is made through the tendon over the joint, resecting the collateral ligaments. Two Kirschner wires (K-wires) are then passed obliquely across the joint to provide stable fixation, usually at 25° of flexion for the index finger and slightly more for the third digit. Postoperative care consists of cast immobilization for 6-8 weeks.
Arthroplasty can be performed if the surrounding soft tissues are adequate. A dorsal incision is made to expose the extensor mechanism and is split longitudinally. The articular surfaces of the opposing proximal and middle phalanges are removed, and the medullary canals are prepared for the insertion of the implant. The skin is closed with the joint in slight flexion. A palmar incision is then made to release any flexor tendon adhesions. Postoperative care includes splinting the PIP joints in 10° or 20° of flexion and instituting passive and active exercises with a dynamic extension/flexion splint.
Boutonniere deformity
The same disease processes in RA that result in swan-neck deformity also cause the converse deformity, the boutonniere, in which the PIP joint is flexed and the DIP and MP joints are hyperextended. This abnormal finger posture usually starts with PIP joint flexion that leads to the changes in the other joints. Specifically, synovial proliferation in the PIP joint stretches and weakens the extensor mechanism; therefore, full extension cannot be maintained. Then, the lateral bands are displaced volarly and the oblique retinacular ligaments are shortened, which causes hyperextension of the DIP joint. In compensation for increasing PIP joint flexion, the MP joint hyperextends. Generally, if the problem is corrected early, passive treatments are adequate. In the later stages, salvage procedures are indicated.
With mild boutonniere deformity, minimal distortion of the joint positions and functional loss occur. A slight extensor lag (10-15°) is present at the PIP joint with slight hyperextension at the DIP joint and no involvement of the MP joint. Surgical treatment usually consists of extensor tenotomy, which should not threaten existing function while increasing DIP joint flexion.
With moderate boutonniere deformity, the flexion deformity at the PIP joint increases to 30-40° and the MP joint begins to hyperextend in order to compensate. At this stage, reconstruction of the extensor mechanism by shortening the central slip and moving the lateral bands dorsally is indicated to restore PIP extension and hand function. Certain criteria should be met before correction is attempted, including good dorsal skin, smooth joint surfaces, functional flexor tendons, and the ability to passively correct the PIP joint. [4] If wrist deformity is present, it should be corrected before addressing the PIP extensor mechanism. Conversely, PIP joint flexion should be corrected before MP joint arthroplasty because achieving adequate MP joint flexion while the PIP joint is also flexed is difficult.
When the PIP joint can no longer be passively extended, the boutonniere deformity is severe. Fusion of the joint or arthroplasty is now warranted. Fusion of the PIP joint should be performed so that the treated finger is in a functional or flexed position. The degree of flexion should increase from the index finger (25°) to small finger (40°). [4] Postfusion, patients often regain MP joint function. If PIP joint arthroplasty is chosen in order to maintain motion, the extensor mechanism should also be repaired. This option is particularly useful for boutonniere deformities of the small and ring fingers to maintain or improve grip strength.
Thumb deformities
The deformities of the thumb that arise from RA are similar yet, in many ways, different from those of the fingers. Significant forces are placed at the IP, MP, and carpometacarpal (CMC) joint of the thumb in daily activities that promote significant degeneration in the face of proliferative synovitis of RA.
Classification of thumb deformities
Nalebuff described 5 types of deformities of the thumb associated with RA, and Ratliff described a sixth type.
Type 1 thumb disorder is a boutonniere deformity. The pathology originates at the MP joint with significant synovitis, dorsal hood disruption, and subluxation of the EPL tendon ulnarly. The distal phalanx is drawn into extension as the proximal phalanx subluxes dorsally. The type 1 deformity is the most common in persons with RA.
Type 2 thumb deformity maintains the hyperextension at the MP joint, but it is also associated with hyperextension at the IP joint. Also, the CMC joint is often subluxed.
The type 3 thumb deformity is a swan-neck deformity and is the second most common type of thumb disfigurement in persons with RA. CMC joint synovitis initiates the subluxation of the first metacarpal radially and dorsally. The first metacarpal is adducted. This adduction combined with volar plate laxity results in hyperextension of the MP joint. A relative shortening of the lateral bands and the pull of the flexor pollicis longus draw the distal phalanx into flexion.
The type 4 deformity is analogous to skier thumb or gamekeeper thumb. The MP joint synovitis initiates the pathology, with resultant laxity of the ulnar collateral ligament with or without subsequent adduction of the first metacarpal.
The type 5 deformity is identical to the type 3 deformity but does not involve adduction of the first metacarpal. The disease in type 5 is initiated at the MP joint, fostering volar plate laxity, subsequent MP hyperextension, and IP joint flexion.
The type 6 deformity involves isolated IP joint and/or MP joint destruction with subluxation, as a result of bone resorption and destruction.
Treatment of thumb deformities
The treatment of these specific deformities depends on the severity of disease in each involved joint. Fixed joint deformities with bony destruction are usually more amenable to fusion than arthroplasty. However, in general, in an attempt to preserve motion, fusion of 2 joints in tandem is considered the last-stage effort. IP joint fusion is tolerated extremely well and maintains function. The IP joint should be fused at 0-20° of flexion. MP joint arthroplasty is recommended at the CMC joint or IP joint about to be fused. This preserves the motion of the thumb for prehensile tasks. Pathology isolated to one joint, such as in a type 4 deformity, can be surgically treated with an arthroplasty or volar plate advancement. A type 6 deformity requires fusion to regain stability. Bone grafts may be required to restore adequate bone stock.
CMC joint arthroplasties are a good option for significant deformities of the thumb if the disease initiated at the CMC joint (ie, type 3 deformities). Ligament reconstruction with tendon interposition offers a stable joint with preservation of motion. Occasionally, fusion of the CMC joint may be required if significant bone destruction is present with poor residual bone stock.
Rheumatoid Arthritis of the Wrist
RA can affect any tendon sheath or joint in the body. Often, distal appendage deformities are the result of the proximal joint pathology. This is no truer than in the wrist. Significant wrist synovitis and destruction results in dysfunctional pain, weakened grip, and distal finger deformities. Therefore, correction of the wrist pathology is usually required before, if not at the time of, MP joint or finger reconstruction efforts.
Pathology
Pathology of RA of the wrist is somewhat predictable and usually follows a progressive pattern. The ulnar side of the wrist is most commonly affected with synovitis that destroys the ulnar carpal stabilizing ligaments. The triangular fibrocartilage complex (TFCC) is attenuated, and the distal radial ulnar joint becomes eroded as the dorsal capsule is disrupted. The proliferative synovitis results in dorsal subluxation of the distal ulna, volar subluxation of the extensor carpi ulnaris tendon, and supination of the proximal carpal bones. These signs are collectively known as caput ulna syndrome.
Destructive synovitis at the radial side of the wrist results in (1) attenuation of the radioscaphocapitate ligament with rotary subluxation of the scaphoid and (2) ulnar translocation of the carpal bones. The carpal height collapses, and bony destruction of the wrist ensues. Clinically, the hand deviates radially and maintains a supinated position. This imbalance causes ulnar drift of the phalanges on the metacarpals as the extrinsic forces of the extensors and flexors pull the fingers into this position. Therefore, the pathology of the wrist promotes distal finger deformity. Correction of the wrist pathology before correction of the fingers is often prudent to prevent recurrences of the finger deformities.
Treatment
Early treatment of synovitis of the wrist consists of synovectomy at the ulnar carpal or radial carpal sites. This procedure may help to control pain, slow the progression of the disease, and maintain motion.
A study by Lee et al indicated that arthroscopic wrist synovectomy can control synovitis in 75% of rheumatoid wrists that have been unresponsive to drug therapy. The study involved 49 patients (56 wrists) with RA who underwent arthroscopic synovectomy, with the patients evaluated for wrist pain and function after a mean 7.9-year follow-up period. There was no recurrence of synovitis in 42 wrists (75%), with the mean visual analogue scale score for wrist pain in the study having dropped from 6.3 to 1.7 and the mean Mayo wrist score (as determined from 39 wrists) having risen from 48 to 76. Patient satisfaction reached a mean visual analogue scale score of 7.9. The mean Larsen score rose from 2.2 to 3.3. [22]
A retrospective observational study by Berhouet et al indicated that in patients with RA of the wrist, combining synovectomy with transfer of the extensor carpi radialis longus to the extensor carpi ulnaris can relieve pain and prevent radiocarpal destabilization. At mean 42.5-month follow-up, the investigators found pain relieved in 14 out of 16 wrists (87.5%), with synovitis resolved in 10 wrists (62.5%). Mean increases in extension and flexion were 19.7° and 5.7°, respectively. Reducible radial deviation and ulnar translocation were cited as the primary indication for extensor carpi radialis longus transfer. [23]
RA of the wrist that has progressed beyond simple proliferative synovitis may require more radical treatments. Destruction of the distal radial ulnar joint may require excision of the distal ulna and reconstruction of the TFCC. Tendon transfer reconstruction of the ruptured extensor tendons often associated with caput ulna syndrome can be performed in the same setting as the resection of the distal ulna and synovectomy.
A few reports have indicated that the Sauvé-Kapandji procedure may be more appropriate in younger patients. [24] This procedure provides a fusion of the distal radius and ulnar head and excision of the ulnar neck. The surgery may be more beneficial in those patients who do not have significant ulnar translocation of their carpus. Stabilizing techniques for the distal ulna following resection include using a segment of the radial carpal volar ligament or slips of the extensor carpi ulnaris or flexor carpi ulnaris tendons. Stabilizing procedures following excision of the distal ulnar head may help to prevent complications such as painful forearm rotation.
A study by Ikeda et al found the Sauvé-Kapandji procedure effective on distal radioulnar joints affected by either RA or osteoarthritis. The investigators reported a significant increase in supination and decrease in palmer flexion in patients with RA, determining at 1-year follow-up that carpal alignment and ulnar stump stability had been well maintained. [25]
With destruction of the radial carpal joint, wrist fusion becomes a very functional and viable option. Numerous methods are used for wrist fusion, including the use of Steinman pins, 62-gauge K-wires, plates, and screws. These techniques require the use of autologous bone graft from the iliac crest or allograft bone material. The exact position the wrist should be in following fusion is controversial. Most surgeons prefer to fuse the wrist at an angle of approximately 10° of dorsal flexion.
A retrospective study by Okabayashi et al indicated that in patients with RA of the wrist, long-term, painless stability can be achieved through radiocarpal arthrodesis. The surgery was performed in combination with synovectomy and the Darrach procedure, with the investigators finding at 20-year follow-up that 16 of 20 wrists (80%) possessed increased average grip power, with decreased grip reported for the rest. Significant decreases were found in wrist extension and flexion, while supination and pronation stayed within the functional range. [26]
Questions & Answers
Overview
When is hand and wrist surgery indicated in the treatment of rheumatoid arthritis (RA)?
What is the role of hand and wrist surgery in the treatment of rheumatoid arthritis (RA)?
Where does hand tenosynovitis occur in rheumatoid arthritis (RA)?
What is the pathophysiology of tenosynovitis in rheumatoid arthritis (RA)?
What is trigger finger in in rheumatoid arthritis (RA)?
How is trigger finger in in rheumatoid arthritis (RA) treated?
What causes tendon rupture in rheumatoid arthritis (RA)?
What is the cardinal sign of tendon rupture in rheumatoid arthritis (RA)?
What are the signs and symptoms of extensor tendon rupture in rheumatoid arthritis (RA)?
How is extensor tendon rupture in rheumatoid arthritis (RA) treated?
How is tendon rupture differentiated from other sequelae of rheumatoid arthritis (RA)?
How is flexor tendon rupture in rheumatoid arthritis (RA) diagnosed?
How is flexor tendon rupture in rheumatoid arthritis (RA) treated?
What causes ulnar drift in rheumatoid arthritis (RA)?
What causes volar dislocation in rheumatoid arthritis (RA)?
How are deformities of the metacarpophalangeal (MP) joint in rheumatoid arthritis (RA) treated?
What is the prevalence of swan-neck deformity in rheumatoid arthritis (RA)?
When is surgery indicated for a swan-neck deformity in rheumatoid arthritis (RA)?
How is a swan-neck deformity in rheumatoid arthritis (RA) assessed?
How are swan-neck deformities in rheumatoid arthritis (RA) classified?
What causes a type I swan-neck deformity in rheumatoid arthritis (RA)?
How is a type I swan-neck deformity in rheumatoid arthritis (RA) treated?
What causes a type II swan-neck deformity in rheumatoid arthritis (RA)?
How is a type II swan-neck deformity in rheumatoid arthritis (RA) treated?
How is a type III swan-neck deformity in rheumatoid arthritis (RA) characterized?
How is a type III swan-neck deformity in rheumatoid arthritis (RA) treated?
How is a type IV swan-neck deformity in rheumatoid arthritis (RA) treated?
How is boutonniere deformity in rheumatoid arthritis (RA) treated?
What causes thumb deformities in rheumatoid arthritis (RA)?
How are thumb deformities in rheumatoid arthritis (RA) classified?
How are thumb deformities in rheumatoid arthritis (RA) treated?
What causes writs synovitis in rheumatoid arthritis (RA)?
What is the pathology of rheumatoid arthritis (RA) of the wrist?
How is rheumatoid arthritis (RA) of the wrist treated?
-
Widespread osteopenia, carpal crowding (due to cartilage loss), and several erosions affecting the carpal bones and metacarpal heads in particular in a child with advanced juvenile rheumatoid arthritis (also known as juvenile idiopathic arthritis).
-
Rheumatoid changes in the hand. Photograph by David Effron MD, FACEP.










