Definition
At its most basic level, a flap is a portion of tissue that can be dissected, elevated, and inset into a nonanatomic position as a consequence of its vascular supply and outflow. By this definition, flaps may be composed of any combination of skin, fascia, muscle, and bone. Complexity may range from a random pattern skin flap based on a subdermal plexus to a fascio-osteocutaneous free flap supplied by a known arterial perforator.
A systematic approach to choosing the appropriate form of soft tissue coverage for a wound defect, commonly referred to as the "reconstructive ladder," was popularized by Mathes and Nahai in 1982. [1] In order of increasing complexity, the "rungs" of the ladder are:
-
Primary closure
-
Secondary intention
-
Skin graft
-
Tissue expansion
-
Local flap
-
Regional flap
-
Free tissue transfer
Free tissue transfer is defined as the vascular dissection and detachment of an isolated and specific region of the body (eg, skin, fat, muscle, bone) and transfer of said tissue to another region of the body, with anastomosis of the divided artery and vein to a separate artery and vein located at the site of the defect. The latter portion of this procedure ensures the perfusion and drainage, and ultimately the survival, of the flap. This ability to transplant living tissue from one region of the body to another has greatly facilitated the reconstruction of complex defects.
Free tissue transfer has become commonplace in many centers around the world. The numerous advantages of this technique include stable wound coverage, improved aesthetic and functional outcomes, minimal donor site morbidity, and the ability to utilize vascularized tissue from remote parts of the body that are outside the zone of injury (trauma, malignancy, infection, irradiation, etc). Since the introduction of free tissue transfer in the 1960s, the success rate has improved substantially, currently being 95-99% among experienced surgeons. This article provides a framework to facilitate the planning, execution, and monitoring of free flaps.
For more information on various flap procedures, see Flaps.
Indications
Free tissue transfer currently is used for the reconstruction of complex defects and disorders throughout the body. As with all techniques in plastic surgery, adherence to the basic principles and concepts of reconstruction is essential. The previously mentioned "reconstructive ladder" allows the plastic surgeon to proceed through a cognitive, step-wise progression of reconstructive options of increasing complexity. Generally speaking, the simplest solution is preferable; however, there are specific instances in which this line of logic is bypassed by the "reconstructive escalator," with a free tissue transfer being deemed the optimal and safest solution.
The hierarchy of needs regarding a wound defect starts at wound coverage, with aesthetic outcome very much a secondary concern. Plastic surgery is rarely binary, with wound coverage and aesthetics infrequently seen as mutually exclusive goals. Therefore, depending on surgical expertise, a combination of aesthetic and functional considerations occasionally warrant performing more complicated procedures. These considerations are most evident following ablative procedures for cancer, for restoration of function, and for aesthetic appearance.
Numerous clinical situations exist in which the use of a free flap is justified and beneficial. Refinements in mandibular reconstructions have led to the use of the free fibular flap, which results in improved appearance and function of the neomandible (see the image below).
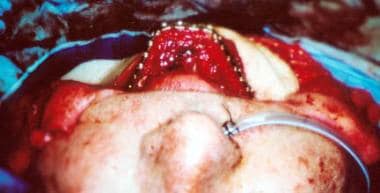 The fibula free flap is well suited to anterior arch defects. The complete arch can be rebuilt following shaping of the bone.
The fibula free flap is well suited to anterior arch defects. The complete arch can be rebuilt following shaping of the bone.
The use of the muscle-sparing free transverse rectus abdominis myocutaneous (TRAM) and deep inferior epigastric (DIEP) artery and vein flaps in breast reconstruction allows for excellent shape and contour of the breast mound while minimizing donor site morbidities related to abdominal strength and contour. [2]
A study by Di Pace et al indicated that in immediate unilateral abdominal free flap breast reconstruction, better outcomes are obtained if the flap weighs approximately as much as or more than the mastectomy tissue. The investigators reported that among patients in whom the flap-to-mastectomy weight ratio was less than one, the rate of contralateral balancing procedures was 37%, versus 11% in those in whom the ratio was more than one and 1% in patients in whom the flap/mastectomy weights were equal. Moreover, in patients in whom the weight ratio was below one, the rates of ipsilateral revision procedures were two and almost three times greater than those of the other groups, respectively. [3]
Innervated free muscle flaps have successfully restored upper extremity and hand function and the ability to generate facial animation in incidents of nerve and muscular dysfunction. The use of numerous perforator flaps, such as the DIEP flap, superficial inferior epigastric artery (SIEA) flap, thoracodorsal artery perforator (TAP) flap, and superior or inferior gluteal artery perforator (SGAP, IGAP) flaps have served to further increase the surgeon's options and further decrease donor site morbidity. [4]
Lymph transfer surgery has been utilized to ameliorate the symptoms and sequelae of chronic peripheral lymphedema. Advancements in microsurgical technique have enabled the use of free lymph node transfers and lymphaticovenous anastomoses to treat lymphedema that has otherwise been recalcitrant to pharmacologic and supportive measures. [5]
Preoperative Considerations
Preoperative preparation is an essential component of the successful free tissue transfer. Preoperative evaluation includes analysis of the recipient site, consideration of available donor sites, and the clinical status of the patient. Proper patient selection is of utmost importance when analyzing outcomes. The specific factors are reviewed below.
Analysis of recipient and donor sites
Factors related to the recipient site include the size, depth, and location of the defect; quality of the surrounding tissue; exposure of vital structures or hardware; zone of injury; presence of bacterial colonization or infection; previous irradiation; [6] and functional and aesthetic considerations. Factors related to the donor site include appropriate tissue match; length of the vascular pedicle; caliber of recipient vessels; surface area, volume, and thickness of the flap; and donor site morbidities.
A basic principle of reconstructive surgery is replacing "like with like." The plastic surgeon must develop a clear concept of the defect to be addressed, ie, whether it is purely a soft tissue deficiency or a composite defect of bone, cartilaginous support, mucosa, etc. Beyond the structure of the defect itself, there may also be a concomitant functional deficit that requires the transfer of innervated muscle tissue, as in the use of a free gracilis flap in the setting of facial reanimation. [7]
Flaps with a short vascular pedicle requiring a vein graft and flaps with a bone component are associated with an increased rate of flap loss in some clinical series. [8] Some sites, such as the head and neck, have various recipient vessel options; therefore, a thorough understanding of the anatomy is essential. [9, 10]
Clinical status of the patient
The clinical status of the patient depends on a variety of factors that also may impact the free flap. These include age, nutritional status, tobacco usage, and the presence of underlying comorbidities (eg, diabetes mellitus, cardiopulmonary disease, peripheral vascular disease). Although advanced age and tobacco use are not contraindications to free-flap operations, poor nutritional status can impede wound healing and recovery. Patients with poorly controlled diabetes mellitus and peripheral vascular disease require adequate glucose control and may need revascularization procedures prior to free tissue transfer. Surgical clearance by a medical physician is recommended for patients with multiple medical problems.
A study by Kwok and Agarwal indicated that in patients who undergo microvascular free tissue transfer, an American Society of Anesthesiologists (ASA) patient classification of 3 or greater and a prolonged operative time are independent risk factors for unplanned reoperations. The study included 2244 patients, 290 of whom underwent unplanned reoperations. [11] Coskunfirat et al evaluated the safety of microvascular free flap surgery for the elderly population, corroborating Kwok and Agarwal's results in finding a heightened risk of postoperative complications for patients who are categorized as ASA class 3 or 4. (However, neither operative time nor age was found to be a risk factor for postoperative complications in this study.) [12]
Donor tissues
Specific donor tissues are variable, and donor sites are chosen based on recipient site requirements. Available tissues include muscle, musculocutaneous, fasciocutaneous, osteocutaneous, and bone flaps. In general, free muscle flaps are indicated for soft tissue coverage of bone and synthetic materials and to obliterate a large dead space. [13, 14]
-
Innervated muscle flaps are useful for facial reanimation operations and for upper extremity reconstruction
-
Musculocutaneous free flaps are useful for large defects requiring aesthetic contouring [15]
-
Fasciocutaneous flaps permit tendon gliding in the extremities and provide excellent contouring of the head and neck
-
Osseous and osteocutaneous free flaps are useful for segmental bone defects involving the mandible and extremities [15]
-
Adipocutaneous or perforator flaps are especially useful to minimize donor site morbidity
-
For the irradiated wound, free tissue transfer is recommended and has been demonstrated to be safe and well tolerated, with no increased rate of partial or total free flap loss
Timing
In the trauma patient, the timing of free-flap reconstruction is of prime importance. Free tissue transfer within 3-7 days allows time for adequate debridement, declaration of the zone of injury, and prevention of chronic bacterial colonization, [16, 17] whereas it was previously thought that delayed reconstruction (more than 72 hours after the injury) independently increased the risk for postoperative infection or flap loss. [18] Immediate free-flap reconstruction is often preferred for the acquired operative wound, especially in the presence of vital structures and hardware and for aesthetic and functional considerations. Consider delayed free-flap reconstruction when oncologic concerns are present.
Other considerations
Other factors that require consideration include choice of anesthesia and patient position for the operation. Anesthetic options include general, spinal, and epidural, with the choice depending on the nature and location of the reconstruction. General anesthesia is preferred for most patients and can be administered via oral, nasal, or tracheal routes. Oral intubation is preferred for trunk and extremity reconstructions; however, nasal and tracheal intubations are preferred for most reconstructions involving the head and neck. Spinal anesthesia occasionally is used for lower extremity free flaps and has the advantage of providing a transient sympathectomy that promotes vascular dilation. Epidural anesthesia is used primarily for postoperative pain management.
Patient positioning may require an inflatable beanbag, Wilson frame, or Mayfield headrest. The inflatable beanbag is useful in placing the patient in the lateral decubitus position (eg, when harvesting a latissimus dorsi flap). The Wilson frame or chest rolls benefit patients in the prone position, allowing chest expansion during general anesthesia.
Intraoperative Considerations
The operative portion of the free tissue transfer requires absolute attention to detail. Numerous factors must be considered to predictably obtain a successful outcome. These include use of appropriate medications and solutions, properly functioning equipment and instruments, anastomotic issues, and flap insetting.
Intraoperative medications
Required medications include intravenous antibiotics, antibiotic solution for wound irrigation, intravenous heparin administered 5 minutes prior to free flap harvest, 4% Xylocaine for topical vasodilatation, and heparin solution (100 U/cm3) for luminal irrigation. Select studies evaluating the effects of various intraoperative anticoagulants have demonstrated that the flap loss rate is lower in patients receiving a heparin bolus of 5000 U only or a heparin bolus of 2000-3000 U followed by postoperative infusion. [19] Low-dose heparin does not increase the risk of hematoma or postoperative bleeding. However, a consensus has yet to be reached regarding the ideal chemoprophylactic regimen to minimize thromboembolic events. Some authors advocate the use of daily aspirin versus ketorolac as an alternative and/or adjunct to heparin or Lovenox therapy. Other medications that may be used include Decadron 4-8 mg to reduce edema and swelling (especially for reconstructions of the head), papaverine as an alternate vasodilator, and streptokinase or urokinase for lysis of intraluminal thrombus.
Anastomoses issues
Various issues are related to the anastomoses. [20] Factors contributing to a difficult anastomosis include trauma to the zone of injury, radiation, scarring, and infection. Success can be amplified by adhering to some basic principles, as follow:
-
The nursing staff and primary surgeon must inspect the microinstruments and microscope to ensure proper function.
-
The diameter of the artery and vein, both for the flap and recipient site, should be 1-3 mm to permit adequate inflow and outflow.
-
Blood vessels must be free of all loose adventitia, and the vascular approximation must be tension free. Acland clamps should facilitate vascular exposure and manipulation.
-
Complete the anastomosis using either a vascular coupler, or sew it by hand. The coupler has demonstrated its usefulness, especially for venous anastomoses, in improving patency and decreasing operative time. [21]
-
Complete the hand-sewn anastomosis using 8-0, 9-0, or 10-0 nylon sutures placed in an interrupted fashion. In general, anastomose larger-caliber vessels (2-3 mm) using 8-0 or 9-0 sutures and smaller-caliber vessels (1-2 mm) using 9-0 or 10-0 sutures.
-
The use of operative loupes rather than a microscope has been reported; a minimum of 3.5-power magnification is recommended. [22]
-
The tolerated flap ischemia times depend upon the composition of the tissues being transferred. In general, perforator flaps tolerate longer periods of ischemia because no muscle is involved. Ischemia times of up to 4 hours for a perforator flap may be well tolerated. Musculocutaneous flaps, on the other hand, do not tolerate prolonged ischemia times because of the metabolic requirements of the muscle. In general, 2-3 hours of ischemia is the maximum time tolerated. [23]
-
Following completion of the anastomoses, the flap must be properly inset. Inspect the vascular pedicle for kinks, twists, and compression and to ensure that no tension is present across the anastomosis. Inspect the distal aspect of the flap for arterial and venous bleeding. Use a Doppler unit to assess arterial and venous flow through the pedicle and in the flap. Finally, recheck the vascular pedicle to ensure that there is a gentle, nontwisting course for the vessels before completing the final suturing of the flap, especially if the patient's position has been changed.
-
Place a closed suction drain under the flap away from the anastomoses and suture the flap in position.
A histologic study by Seo et al of failed microvascular free flap procedures revealed damage to the endothelium of microanastomosed vessels and degeneration of smooth muscle beneath the endothelium. The investigators suggested that the endothelial damage and exposure of connective tissue elements during microanastomosis trigger thrombosis at the microanastomotic sites. [24]
Postoperative medications
Using postoperative medications to inhibit clot formation at the anastomosis is controversial. Studies evaluating the efficacy of heparin, dextran, and aspirin have demonstrated that none of these medications is absolutely necessary for an uncomplicated anastomosis. A report by Ashjian et al demonstrated no difference in outcome when a daily full-dose aspirin regimen was compared with the use of prophylactic low–molecular-weight heparin, with regard to thromboembolism, bleeding, or flap loss. [25]
Postoperative Monitoring
Frequent postoperative evaluation remains the gold standard for assessing flap viability. [26] Techniques to monitor the free flap depend on the tissue composition and location of the flap. Specific characteristics to track include color, capillary refill, turgor, surface temperature, presence of bleeding, skin graft adherence, and auditory assessment of blood flow. Adjunctive measures for flap monitoring include the use of pulse oximetry, external Doppler ultrasonography, external cutaneous paddles (for buried flaps), laser Doppler flowmeter devices, and pulsed Doppler ultrasonography. [27] Use of these techniques depends on whether the flap has a fasciocutaneous component, is covered with a skin graft, or is buried and inaccessible to visual assessment.
Surface characteristics
For the fasciocutaneous, adipocutaneous, musculocutaneous, and osteocutaneous flaps, surface characteristics are important. Normal flap color is similar to that of the recipient site. Normal capillary refill is 1-2 seconds. Surface temperature of the flap can be monitored using adhesive strips (for an accurate number) or the back of the hand (to provide a comparative assessment with the surrounding skin), although the latter would require serial assessments by the same examiner. Problems with arterial inflow are suggested when the flap is pale relative to the donor site and cool to the touch and when capillary refill is delayed or absent. Problems with venous outflow are suggested when the flap is congested and edematous and when capillary refill is brisk and rapid. Color and appearance of congested flaps can vary depending on whether the congestion is mild or severe and ranges from a prominent pinkish hue to a dark bluish purple color.
Surface Doppler assessment for flaps with a fasciocutaneous component may yield a false-positive result by picking up signals from surrounding or deep blood vessels. Characteristics of blood from the flap following pinprick also can provide clues. Dark venous blood suggests venous occlusion, and absence of bleeding suggests arterial occlusion.
Muscle flaps with skin grafts
The muscle flap covered with a skin graft often is easier to monitor. Surface temperature and capillary refill generally are not used in these situations; however, color, turgor, skin graft adherence, and Doppler signals are useful. Signs of venous outflow obstruction include flap congestion and edema, dark blood on pinprick, and loss of the venous Doppler signal. Signs of arterial occlusion include a flat and pale flap, poor skin graft adherence to the flap, no bleeding on pinprick, and loss of the arterial signal.
Deep or buried flap
The most difficult flap to monitor is the deep or buried flap (eg, fibula flap without a skin paddle). Surface Doppler signals often are unreliable. In these situations, placing a temporary, implantable Doppler probe adjacent to the artery and vein at the time of operation is useful. Recently, implantable tissue oximetry has been utilized as an adjunctive measure in assessing flap perfusion. [28, 29]
Salvage Procedures for the Failing Flap
Monitoring the free flap during the postoperative phase is critical to ensuring flap survival. When recognized early and managed promptly (< 6 h), compromised flaps have a 75% salvage rate when taken back to the operating room. Adipocutaneous flaps can tolerate ischemia better than musculocutaneous flaps can. Studies have demonstrated that venous thrombosis alone is more common than either arterial or combined arterial and venous thrombosis. [30] Thrombosis typically occurs within the first 2 days in 80% of patients. Thus, all personnel responsible for flap monitoring must be knowledgeable about the appearance and evaluation of the healthy and compromised flap. Other points to remember include the following:
-
Following recognition of flap compromise, immediately transport the patient to the operating room for exploration.
-
Administer intravenous heparin.
-
Inspect the vascular pedicle for kinks and compression and assess the patency of the anastomosis.
-
Identification of thrombus requires separation of the vessels at the anastomosis.
-
Perform embolectomy proximally and distally, using a number 2 or 3 Fogarty catheter.
-
Administer intra-arterial streptokinase or urokinase at a dose of 50,000-100,000 U as necessary.
-
Following restoration of adequate circulation, inset the flap again and maintain the patient on intravenous heparin or dextran.
-
Failure to restore adequate circulation requires flap removal.
-
The use of medicinal leeches, Hirudo medicinalis, has demonstrated value in the treatment of venous congestion. [31] This option is indicated when arterial inflow is adequate but venous outflow is poor. The mechanism of action depends on the active agent produced by the leeches, hirudin, which is a selective thrombin inhibitor. Apply the leech to the surface of the flap and surround it with a corral of moistened gauze to prevent leech migration. Prophylactic antibiotics are recommended to prevent infection with Aeromonas hydrophila. [32]
Summary
Free tissue transfer represents an adaptable tool within the armamentarium of the plastic surgeon for the coverage of complex tissue defects. Considerable thought must be invested, however, prior to this surgical intervention, including with regard to the nature of the defect, the clinical status of the patient, the technical abilities of the surgeon, the ability of the operating facility to provide adequate postoperative care and flap monitoring, and, most importantly, whether a simpler solution exists that could produce a comparable reconstructive outcome. As the highest rung on the reconstructive ladder, free tissue transfer is an incisive tool that must be used judiciously.
-
The fibula free flap is well suited to anterior arch defects. The complete arch can be rebuilt following shaping of the bone.









