Practice Essentials
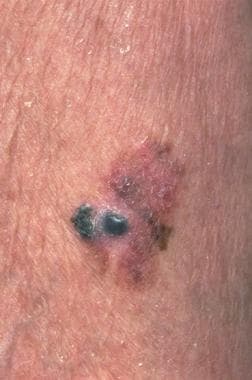
A melanoma is a tumor produced by the malignant transformation of melanocytes. Melanocytes are derived from the neural crest; consequently, melanomas, although they usually occur on the skin (see the image below), can arise in other locations where neural crest cells migrate, such as the gastrointestinal tract and brain. The 5-year relative survival rate for patients with stage 0 melanoma is 97%, compared with about 10% for those with stage IV disease.
Signs and symptoms
The characteristics of melanoma (known by the acronym ABCDE) include the following:
-
A: Asymmetry
-
B: Irregular border
-
C: Color variations: Especially red, white, and blue tones in a brown or black lesion
-
D: Diameter greater than 6 mm
-
E: Elevated surface
In addition, melanomas may itch, bleed, ulcerate, or develop satellites. Patients who present with metastatic disease or with primary sites other than the skin have signs and symptoms related to the affected organ system(s).
Histology
Histologic classification
The 4 major types of melanoma, classified according to growth pattern, are as follows:
-
Superficial spreading melanoma: Constitutes approximately 70% of melanomas; usually flat but may become irregular and elevated in later stages; the lesions average 2 cm in diameter, with variegated colors, as well as peripheral notches, indentations, or both
-
Nodular melanoma: Accounts for approximately 15-30% of melanoma diagnoses; the tumors typically are blue-black but may lack pigment in some circumstances
-
Lentigo maligna melanoma: Represents 4-10% of melanomas; the tumors are often larger than 3 cm, flat, and tan, with marked notching of the borders; they begin as small, frecklelike lesions
-
Acral lentiginous melanoma: Constitutes 2-8% of melanomas in Whites and 35-60% of them in dark-skinned people; may appear on the palms and soles as flat, tan, or brown stains with irregular borders; subungual lesions can be brown or black, with ulcerations in later stages
Biopsy
Perform excisional biopsy on suggestive lesions. The most important prognostic indicator for stage I and II tumors is thickness, so a full-thickness biopsy must be obtained for adequate pathologic interpretation.
Management
Surgical therapy for melanoma, as follows, is based on the predicted risk of local recurrence and metastatic disease and the potential morbidity of the operation:
-
Stage 0: Widely excise the tumor or previous biopsy site; use a 0.5- to 1-cm margin for melanomas in situ
-
Stage I: 1-cm excision margins are adequate, but lesions greater than 1 mm require 2-cm margins; for lesions with a depth greater than 1 mm, many authorities recommend sentinel lymph node biopsy at the time of wide local excision
-
Stage II: Perform a 2-cm surgical resection; carry out a complete therapeutic lymphadenectomy on patients with suspected lymph node metastases based on physical examination findings; consider sentinel lymph node biopsy if no clinically positive nodes are present
-
Stage III: Wide local excision of the primary tumor with 2-cm margins remains first-line therapy; [1] perform regional lymph node dissection because a stage III melanoma represents nodal disease; If the nodal status is unknown, consider a sentinel lymph node biopsy to determine if the disease is stage I, II, or III
-
Stage IV: Usually refractory to standard therapy; thus, consider these patients for clinical trials; surgical resection of isolated metastases in the gastrointestinal tract, the brain, the lungs, or bone may be performed for palliation; metastatic lymph nodes also may be removed for palliation; radiation may provide symptomatic relief for metastases to bone, the brain, or viscera
Overview
History
Melanoma is a tumor that develops as a result of the malignant transformation of melanocytes. These cells are derived from the neural crest. Melanomas usually occur on the skin but can arise in other locations where neural crest cells migrate, such as in the gastrointestinal tract or brain. Melanoma predominantly affects adults, with a peak incidence in the fourth decade, and has no sex prevalence. A patient's risk of developing a second primary melanoma after diagnosis of the first one is 3-5%.
Definition of problem
Melanoma poses an increasingly difficult problem as more people are affected. The incidence is estimated to be rising by almost 6% per year. Recognition of this disease as an entity is crucial so that people may seek medical attention while the tumor is still in its early stages, prior to metastasis. Efforts should be directed toward public awareness campaigns.
Frequency
In the United States, the incidence of melanoma continues to increase, with the prevalence of trunk and extremity lesions rising relatively faster than that of head and neck lesions; however, survival rates are improving.
According to American Cancer Society (ACS) estimates, approximately 100,640 new melanoma diagnoses will be made in the United States in 2024, and about 8290 people will die from the cancer. [2]
As calculated using age-adjusted rates, there was an average annual 1.2% rise in the rate of new cases of melanoma of the skin between 2010 and 2019. However, between 2011 and 2020, the age-adjusted death rate dropped by 3.3% annually, on average. [3]
Currently, in the United States, approximately 1 in 33 White persons, 1 in 1000 Black persons, and 1 in 200 Hispanic persons develops melanoma at some point in his or her lifetime. [2]
Internationally, incidence varies worldwide. A study by Arnold et al found that globally, in 2020, the highest incidence of cutaneous melanoma was in Australia and New Zealand, being 42 per 100,000 person-years (PY) in males and 31 per 100,000 PY in females. The next-highest incidence was in Western Europe, at 19 per 100,000 PY in both males and females. This was followed by North America (18 per 100,000 PY in males and 14 per 100,000 PY in females) and Northern Europe (17 per 100,000 PY in males and 18 per 100,000 PY in females). Melanoma rates were lowest in most African and Asian regions, being below 1 per 100,000 PY, except in middle and southern Africa and in western Asia. [4, 5]
A study by Sacchetto et al found that invasive and in situ melanoma each increased in incidence in Europe over a 17-year period. Using information from 18 European cancer registries, the report found that between 1995 and 2012, the incidence of invasive melanoma cases underwent an average annual percentage change (AAPC) of 4.0% in men and 3.0% in women, while the incidence of in situ melanoma cases underwent an AAPC of 7.7% in men and 6.2% in women. The study also found that the incidence of thin and thick melanomas had increased in incidence over the same time period, with the rise in the incidence of invasive lesions being primarily associated with thin melanomas. [6]
Etiology/risk factors
See the list below:
-
Family history - Positive family history in 5-10% of patients; with at least one affected relative, 2.2-fold higher risk
-
Personal characteristics - Blue eyes, fair and/or red hair, pale complexion; skin reaction to sunlight (easily sunburned); freckling; benign and/or dysplastic melanocytic nevi (number has better correlation than size); immunosuppressive states (transplantation patients, hematologic malignancies)
-
Sun exposure over lifetime - High UVB and UVA radiation exposure (Recent evidence has shown that the risk of melanoma is higher in people who use sunscreen. Because sunscreen mostly blocks UVB, people using sunscreen may be exposed to UVA more than the general public, provided those people are exposed to the sun more than the general public. [7] ); low latitude; number of blistering sunburns; use of tanning beds [8]
-
Atypical mole syndrome (formerly termed B-K mole syndrome, dysplastic nevus syndrome, familial atypical multiple mole melanoma) - Over 10 years, 10.7% risk of melanoma (vs 0.62% of controls); higher risk of melanoma depending on number of family members affected (nearly 100% risk if 2 or more relatives have dysplastic nevi and melanoma)
-
Socioeconomic status - Lower socioeconomic status may be linked to more advanced disease at the time of detection. One survey of newly-diagnosed patients found that low SES-individuals have decreased melanoma risk perception and knowledge of the disease. [9]
Pathophysiology
Some researchers have suggested that benign melanocytic nevi are markers of melanoma risk rather than direct precursors; however, dysplastic nevi are believed to degenerate over time into melanoma. Lentigo maligna is believed to be a preinvasive precursor of lentigo maligna melanoma, and at least 5% progress to malignancy. [10, 11]
Clinical presentation
Patients usually present with skin lesions that have changed in size, color, contour, or configuration. The acronym "ABCDE" is the hallmark of international public awareness campaigns and may be used to remember the physical characteristics suggestive of malignancy. ABCDE stands for asymmetry, irregular border, color variations (especially red, white, and blue tones in a brown or black lesion), diameter greater than 6 mm, and elevated surface. Lesions may itch, bleed, ulcerate, or develop satellites. See images below for examples.
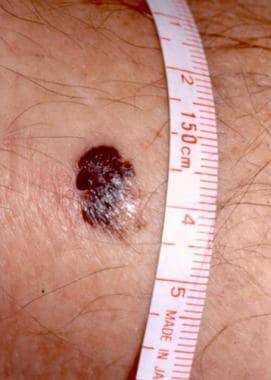 A 1.5-cm melanoma with characteristic asymmetry, irregular borders, and color variation. Courtesy of Wendy Brick, MD.
A 1.5-cm melanoma with characteristic asymmetry, irregular borders, and color variation. Courtesy of Wendy Brick, MD.
Perform excisional biopsy on these suggestive lesions so that a pathologist can confirm the diagnosis. Shave biopsies and electrodesiccation are inadequate; a full thickness of skin is essential for proper histologic diagnosis and classification. The most important prognostic indicator for stage I and II tumors is thickness; obtain a full-thickness biopsy specimen for adequate pathologic interpretation. Biopsy results ultimately determine the margins of resection and which patients are candidates for sentinel lymph node biopsy and other adjuvant treatment.
Patients who present with metastatic disease or with primary sites other than the skin have signs and symptoms related to the affected organ system(s).
Indications and Guidelines
Perform biopsy on all lesions suggestive of melanoma; sample the thickest part of the lesion. Maintain a low threshold to perform biopsy because the procedure is easy and safe. If the resection will not result in a disfiguring defect, excisional biopsy with a 2-mm skin margin and extension to the subcutaneous tissue is suggested for lesions less than 1.5 cm in diameter. Take care to leave scars in locations where re-excision is not difficult. If the lesion is large or located in an anatomic area where skin removal would cause disfigurement, an incisional biopsy may be performed. A full-thickness core punch biopsy in the most raised or irregular area is suggested, with the understanding that this area may not be the thickest area.
Contraindications
Biopsy of a suggestive lesion has no contraindications. Even with widely metastatic melanoma, local control can be attempted.
American Academy of Dermatology clinical practice guidelines
Guidelines released in November 2018 by the American Academy of Dermatology on the evaluation and management of primary cutaneous melanoma include the following [12] :
-
Evidence strongly indicates that Breslow thickness, ulceration, and dermal mitotic rate are important predictors of patient outcome in primary cutaneous melanoma
-
The recommended first-line treatment for any-thickness primary cutaneous melanoma, as well as for melanoma in situ, is surgical excision with histologically negative margins; tumor thickness should dictate the margins
-
Surgical margins for invasive cutaneous melanoma, as measured clinically around the primary tumor, should be a minimum of 1 cm and a maximum of 2 cm, although narrower margins can be employed to accommodate function and/or anatomic location; it is recommended that the excision be as deep as, but not inclusive of, the fascia
-
It is not recommended that asymptomatic patients with newly diagnosed stage 0-II primary cutaneous melanoma undergo baseline radiologic imaging and laboratory studies
-
For cutaneous melanoma at baseline, radiologic imaging and laboratory studies should be conducted only to assess the specific signs or symptoms of synchronous metastasis (regional nodal or distant)
-
At baseline or when physical examination of lymph nodes is equivocal and requires surveillance, the employment of lymph node ultrasonography is encouraged; surveillance with such imaging is also encouraged in patients who meet criteria for sentinel lymph node biopsy (SLNB) but do not undergo the procedure, in patients in whom SLNB is not possible or is technically unsuccessful (eg, because lymphoscintigraphic dye migration has failed and a draining sentinel lymph node cannot be identified), and in those in whom, despite a positive SLNB, complete lymph node dissection is not performed
-
Regular clinical follow-up represents the most important strategy for detecting cutaneous melanoma recurrence; the need for further radiologic or laboratory studies to detect local, regional, or distant metastatic disease should be determined via history (review of systems) and physical examination
-
Patients should be taught self-examination of the skin and lymph nodes in order to detect recurrent disease or new primary cutaneous melanoma
-
For the first 3 months of BRAF inhibitor monotherapy, patients with numerous squamoproliferative neoplasms should undergo dermatologic evaluation every 2-4 weeks, although less skin toxicity is associated with the standard treatment, combination BRAF/MEK inhibition
-
Patients being treated with immune checkpoint inhibitors should undergo dermatologic evaluation within the first month of therapy, with such assessment being continued as needed to manage dermatologic side effects
EXPeRT/PARTNER recommendations
In 2021, the European Cooperative Study Group for Pediatric Rare Tumors (EXPeRT) and the Paediatric Rare Tumours Network - European Registry (PARTNER) published recommendations on the diagnosis and treatment of cutaneous melanoma in children and adolescents. Primary recommendations include the following [13] :
-
Using multidisciplinary teams, members of which include pediatric oncologists and specialists in adult melanoma, to discuss pediatric patients
-
Enrolling patients in available prospective trials
-
Gathering information in national-international databases
-
Creating international collaboration between pediatric and adult melanoma groups so that new agents might better find their way from adult to pediatric care
Relevant Anatomy
The skin is composed of multiple layers. The epidermis is the most superficial layer, and it contains keratinocytes in various stages of development. Melanocytes are located in the deepest layer of the epidermis. A basement membrane separates the epidermis from the underlying dermis, which is divided into 2 zones, papillary dermis and reticular dermis. Subcutaneous tissue is deep to the reticular dermis.
In 1874, Sappey performed an anatomic study of cutaneous lymphatic drainage, which is the foundation for current knowledge of this subject. [14] Sappey's results demonstrated the lymphatic drainage based on anatomic location. His findings were modified later, but then the advent of lymphoscintigraphy led to more accurate lymphatic mapping. Norman et al then redefined the lymphatic basins in a definitive report. [15] They concluded that an extensive overlap of basins drains the head, neck, shoulders, and trunk and that a specific basin cannot be predicted based on cutaneous location. Perform lymphoscintigraphy to define the exact lymphatic drainage for each patient.
Histologic Classification
Melanomas are classified into 4 major types based on growth pattern. They are superficial spreading melanoma, nodular melanoma, lentigo maligna melanoma, and acral lentiginous melanoma. Other more unusual types include mucosal lentiginous melanoma, desmoplastic melanoma, and verrucous melanoma.
Superficial spreading melanoma
Superficial spreading melanomas constitute approximately 70% of melanomas. Histologically, the characteristic cells can be present singly or in nests along the dermal-epidermal junction, but they also may migrate into the stratum granulosum or stratum corneum. These cells can invade the papillary dermis with an inflammatory lymphocytic infiltrate.
Clinically, they usually arise in a preexisting dysplastic nevus. [16, 17] Typically, this lesion changes slowly over several months to years. They are usually flat but may become irregular and elevated in later stages. The lesions average 2 cm in diameter with variegated colors and peripheral notches, indentations, or both.
Nodular melanoma
Histology of nodular melanomas is characterized by extensive vertical growth into the dermis with a minimal radial component. They comprise approximately 15-30% of melanoma diagnoses. These tumors typically are blue-black but may lack pigment in some circumstances. They are known to arise without a preexisting lesion.
Lentigo maligna melanoma
Lentigo maligna melanomas represent 4-10% of melanomas. On a cellular level, dermal and epidermal changes from sun exposure must be present. The histologic appearance is one of irregularly shaped hyperchromatic cells that form spindle-shaped nests. The epidermis is atrophic, while the dermis contains solar elastosis with chronic inflammatory infiltrates. From a clinical standpoint, lentigo maligna melanomas are often larger than 3 cm, flat, tan, and begin as small, frecklelike lesions (see image below). They occur in sun-exposed areas (eg, face and neck of older individuals). Marked notching of the borders is present. Lentigo maligna melanoma usually arises within a Hutchinson freckle (lentigo maligna). When tumor thickness and location are taken into consideration, the prognosis for these melanomas is not believed to be worse than that for other subtypes. [18]
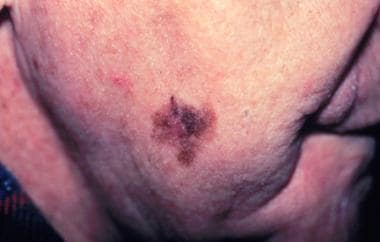 Lentigo maligna melanoma, right lower cheek. Centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Courtesy of Susan Swetter, MD.
Lentigo maligna melanoma, right lower cheek. Centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Courtesy of Susan Swetter, MD.
Acral lentiginous melanoma
This tumor comprises 2-8% of melanomas in Whites and 35-60% of melanomas in dark-skinned people. Cellular proliferation is present along the dermal-epidermal junction with microinvasion into the papillary dermis. The cells have increased melanin granule production, which fills their dendritic extensions. Acral lentiginous melanomas occur on the palms of the hands, beneath the nail beds, and on the soles of the feet. They may appear on the palms and soles as flat, tan, or brown stains with irregular borders. Subungual lesions can be brown or black, with ulcerations in later stages. Although previously no correlation with a worse prognosis was demonstrated for these lesions when tumor thickness was considered, a study by Asgari et al of 123 acral lentiginous melanomas indicated that in patients with these neoplasms, worse melanoma-specific survival can be linked to greater tumor thickness (Breslow depth of over 2 mm), as well as to more advanced tumor stage at presentation. [19]
Desmoplastic melanoma
These lesions account for approximately 1% of melanoma cases; they are fairly rare. They demonstrate a tendency for perineural invasion, especially in the head and neck. They have a propensity for higher local recurrence rates but lower regional metastasis rates.
Classification and Staging
Two classification schemes have been developed, based on either the vertical thickness of the lesion in millimeters or the anatomic level of invasion of the layers of skin. The Breslow classification scheme is used almost exclusively now because it more accurately predicts future tumor behavior. The Clark level is now used only in the staging of thin (T1) melanomas. The TNM (tumor, node, metastasis) system is used for clinical staging as designated by the American Joint Committee on Cancer (AJCC) staging system. [20]
Breslow classification
See the list below:
-
Thickness of 0.75 mm or less
-
Thickness of 0.76-1.5 mm
-
Thickness of 1.51-4 mm
-
Thickness greater than 4 mm
Clark classification
See the list below:
-
Level I - Involves only epidermis (in situ melanoma); no invasion
-
Level II - Invades papillary dermis but not papillary-reticular dermal interface
-
Level III - Invades and expands papillary dermis up to the interface with, but not into, reticular dermis
-
Level IV - Invades reticular dermis but not into subcutaneous tissue
-
Level V - Invades into subcutaneous tissue
TNM staging
Primary tumor (pT)
-
pTX - Primary tumor cannot be assessed
-
pT0 - No evidence of primary tumor
-
pTis - Melanoma in situ; involves only epidermis (CL I)
-
pT1 - Tumor 1 mm or less in thickness; invades papillary dermis (CL II) (or to papillary-reticular dermal interface (CL III) (pT1b can mean that either the melanoma is ≤ 1 mm with ulceration or is < 1 mm but is Clark level IV or V with or without ulceration.)
-
pT2 - Tumor 1.01-2 mm in thickness
-
pT3 - Tumor 2.01-4 mm in thickness
-
pT4 - Tumor greater than 4 mm in thickness and/or invades subcutaneous tissue (CL V) and/or satellite(s) within 2 cm of the primary tumor
-
pT4a - Tumor greater than 4 mm in thickness with or without ulceration
-
Any Ta - Not ulcerated
-
Any Tb - Ulcerated
Regional lymph nodes (N)
-
NX - Regional lymph nodes cannot be assessed
-
N0 - No regional lymph node metastasis
-
N1 - Metastasis in 1 lymph node
-
N2 - Metastasis in 2-3 lymph nodes or spread of melanoma in the skin toward a nearby lymph node area
-
N3 - Metastasis in 4 or more lymph nodes or spread of melanoma in the skin toward a lymph node area and into the lymph node(s)
-
Any Na - Melanoma only seen under the microscope
-
Any Nb - Melanoma in the lymph node visible to naked eye
Distant metastasis (M)
-
MX - Distant metastasis cannot be assessed
-
M0 - No distant metastasis
-
M1 - Distant metastasis
-
M1a - Metastases to skin or subcutaneous (below the skin) tissue or distant lymph nodes
-
M1b - Metastases to lung
-
M1c - Metastases to other organs
AJCC groupings [20]
-
Stage 0 (pTis, N0, M0): The melanoma is in situ, meaning that it involves the epidermis but has not spread to the dermis (lower layer).
-
Stage IA (pT1a, N0, M0): The melanoma is less than 1 mm in thickness and Clark level II or III. It is not ulcerated, appears to be localized in the skin, and has not been found in lymph nodes or distant organs.
-
Stage IB (pT1b or pT2a, N0, M0): The melanoma is less than 1 mm in thickness and is ulcerated or Clark level IV or V, or it is 1.01-2 mm and is not ulcerated. It appears to be localized in the skin and has not been found in lymph nodes or distant organs.
-
Stage IIA (pT2b or pT3a, N0, M0): The melanoma is 1.01-2 mm in thickness and is ulcerated, or it is 2.01-4 mm and is not ulcerated. It appears to be localized in the skin and has not been found in lymph nodes or distant organs.
-
Stage IIB (pT3b or pT4a, N0, M0): The melanoma is 2.01-4 mm in thickness and is ulcerated, or it is thicker than 4 mm and is not ulcerated. It appears to be localized in the skin and has not been found in lymph nodes or distant organs.
-
Stage IIC (pT4b, N0, M0): The melanoma is thicker than 4 mm and is ulcerated. It appears to be localized in the skin and has not been found in lymph nodes or distant organs.
-
Stage III (any pT, N1-3, M0): The melanoma has spread to lymph nodes near the affected skin area. No distant spread is present. The thickness of the melanoma is not a factor, although it is usually thick in people with stage III melanoma.
-
Stage IV (any pT, any N, any M1): The melanoma has spread beyond the original area of skin and nearby lymph nodes to other organs, such as the lungs, liver, or brain, or to distant areas of the skin or lymph nodes. Neither the lymph node status nor thickness is considered, but in general, the melanoma is thick and has spread to lymph nodes.
Treatment & Management
Medical Therapy
Medical therapy is used as adjuvant treatment in advanced stages of unresectable or metastatic melanoma and, most recently, for resected, advanced-stage disease. Approvals from the US Food and Drug Administration (FDA) include trametinib (Mekinist), dabrafenib (Tafinlar), ipilimumab (Yervoy), vemurafenib (Zelboraf), pembrolizumab (Keytruda), nivolumab (Opdivo), and nivolumab/relatlimab (Opdualag). Trametinib is a MEK inhibitor indicated for melanoma with BRAF V600E or V600K mutations. Dabrafenib is a BRAF protein kinase inhibitor indicated for melanoma with BRAF V600E mutation. Ipilimumab is a targeted T-cell antibody that binds to CTLA-4. Vemurafenib is an inhibitor of some mutated forms of BRAF serine-threonine kinase, including BRAF V600E. Pembrolizumab and nivolumab are monoclonal antibodies to programmed cell death-1 (PD-1) protein. They block the interaction between PD-1 and its ligands (ie, PD-L1 and PD-L2). [21, 22]
The fixed-dose combination of nivolumab/relatlimab (Opdualag) is indicated for the treatment of unresectable or metastatic melanoma in adults and pediatric patients aged 12 years or older. Nivolumab is a programmed death receptor-1 (PD-1) blocking antibody, and relatlimab is a lymphocyte activation gene-3 (LAG-3) blocking antibody. The combination increases T-cell activation more than either antibody alone would.
Approval in March 2022 was based on the RELATIVITY-047 phase 2/3 global trial. In the study, the 355 patients who were randomly assigned to the combination therapy achieved a median progression-free survival (PFS) or 10.1 months, while the 359 patients who received nivolumab alone had a median PFS of 4.6 months. [23]
The combination of binimetinib (Mektovi), an MEK inhibitor, plus encorafenib (Braftovi), a BRAF inhibitor, was approved by the FDA in June 2018 for patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation. Approval followed the success of the phase 3 COLUMBUS trial, in which, compared with treatment with vemurafenib alone, binimetinib/encorafenib therapy led to double the median progression-free survival time (7.3 mo vs 14.9 mo, respectively). [24]
A study by Hecht et al indicated that patients with BRAF-mutated melanoma have better overall survival and experience less toxicity when vemurafenib treatment is interrupted during radiotherapy instead of administered concomitantly. For patients who underwent interrupted treatment, median overall survival from the start of radiotherapy and from the beginning of vemurafenib treatment was 10.1 and 13.1 months, respectively, compared with 6.6 and 10.9 months, respectively, for the group that received concomitant therapy. Moreover, there was a greater incidence of skin toxicity of grade 2 or higher (based on Common Terminology Criteria for Adverse Events [CTCAE]) in the concomitant patients than in the interrupted group. [25]
In October 2015, the FDA approved talimogene laherparepvec (Imlygic), a genetically modified, live attenuated herpes simplex virus programmed to replicate within tumors and manufacture granulocyte-macrophage colony-stimulating factor (GM-CSF), an immunostimulatory protein. The first oncolytic viral therapy approved by the FDA, talimogene laherparepvec is indicated for the local treatment of unresectable cutaneous, subcutaneous, and nodal lesions in cases of postsurgery melanoma recurrence. It is administered by injection into lesions that are visible, palpable, or detectable by ultrasonographic guidance. [26]
Approval was based on results from OPTiM (OncoVEX [GM-CSF] Pivotal Trial in Melanoma), a randomized, controlled study conducted in 436 adults with unresectable regionally or distantly metastatic melanoma. In the trial, 295 patients were treated with talimogene laherparepvec, while 141 patients received with GM-CSF. Those who were treated with talimogene laherparepvec had a 16.3% durable response rate (the rate of complete response plus partial response beginning within the first 12 months and lasting continuously for ≥6 months), compared with 2.1% in patients given GM-CSF. A complete response was obtained in 32 patients (10.8%) treated with talimogene laherparepvec, versus just one patient (0.7%) who received GM-CSF. [26, 27]
A retrospective study by Wang et al indicated that patients with advanced BRAF-mutated melanoma have better brain metastasis–free survival (BMFS) and greater overall survival with first-line immunotherapy than with first-line targeted therapy. Patients who underwent first-line immunotherapy had a median BMFS of 41.9 months, compared with 11.0 months for first-line targeted therapy patients, while median overall survival was 48.3 and 13.8 months, respectively. Moreover, first-line targeted therapy was associated with a larger cumulative incidence of brain metastases than was first-line immunotherapy (P < 0.001). [28]
Adjuvant treatment for resected melanoma
In addition to its use against unresectable or metastatic melanoma, in December 2017 nivolumab received FDA approval as an adjuvant therapy for patients who have undergone complete resection of melanoma with lymph node involvement or metastatic disease. Approval stemmed from the CheckMate-238 trial. In that study, investigators found that in completely resected patients with stage IIIB/C or IV melanoma, those treated with nivolumab had a higher 12-month recurrence-free survival rate than did patients treated with ipilimumab (70.5% vs 60.8%, respectively). [29]
Pembrolizumab gained FDA approval in February 2019 for adjuvant treatment of resected, high-risk stage 3 melanoma. Approval was based on data from a phase 3, double-blind trial showing the rate of 1-year recurrence-free survival to be significantly greater with pembrolizumab than with placebo (75.4% vs 61%, respectively). [30]
In December 2021, the FDA approved pembrolizumab for the adjuvant treatment of stage IIB or IIC melanoma following complete resection in adult and pediatric patients aged 12 years or older. Additionally, adjuvant treatment with pembrolizumab for stage III melanoma following complete resection was expanded to include pediatric patients aged 12 years or older. [31]
Approval was based on the phase 3 KEYNOTE-716 trial, in which pembrolizumab patients showed a statistically significant improvement in recurrence-free survival, with the risk of disease recurrence or death reduced by 35%, compared with placebo patients. However, neither group reached median recurrence-free survival. [32]
Autologous tumor-infiltrating lymphocytes (TILs)
Lifileucel (Amtagvi) is a preparation of autologous tumor-infiltrating lymphocytes (TILs) selected on the basis of antigen specificity and tumor reactivity. Upon reintroduction of lifileucel into the patient, the TILs re-infiltrate the tumor, specifically recognize the tumor-associated antigens (TAAs), and initiate tumor cell lysis. Following lifileucel infusion, interleukin 2 (aldesleukin) is administered to support TIL expansion in vivo.
Lifileucel is the first autologous T-cell therapy approved for solid-tumor cancer. It is indicated for unresectable or metastatic melanoma in adults previously treated with a PD-1 blocking antibody and, if the patient is BRAF V600 mutation positive, a BRAF inhibitor with or without a MEK inhibitor.
Accelerated approval stemmed from results of the C-144-01 clinical trial. An objective response rate (ORR) of 31.4% was found among the 153 trial patients who underwent lifileucel treatment, with eight patients demonstrating a complete response, and 40, a partial response. Prior to receiving lifileucel, a median of 3.0 lines of therapy had been administered to the study’s patients, with 81.7% of them having undergone treatment with both anti-PD-1 and anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4). [33]
Surgical Therapy
Surgical therapy for melanoma is based on the predicted risk of local recurrence and metastatic disease and the potential morbidity of the operation. If the lesion has not spread beyond the primary site, it is potentially curable. Most of these lesions are thin (< 1 mm or CL I or II).
Stage 0
After making the diagnosis, widely excise the tumor or previous biopsy site. Use a 0.5- to 1-cm margin for melanomas in situ. No further therapy other than observation for nodal or recurrent disease is necessary.
Stage I
For a T1 lesion, 1-cm excision margins are adequate, but lesions greater than 1 mm require 2-cm margins. Studies demonstrate no improvement in recurrence or survival rates with larger margins of resection. Attempt primary closure and perform skin grafting or flap closure if necessary. For lesions with a depth greater than 1 mm, many authorities recommend sentinel lymph node biopsy at the time of wide local excision (see Stage II below).
Stage II
Perform a 2-cm surgical resection on stage II lesions. No recurrence or survival advantage is gained when 2-cm margins are compared with wider margins (4-6 cm), as has been confirmed in a 2011 European study. [34] Smaller resection decreases the need for skin grafting and inpatient hospital stay. [35]
Perform a complete therapeutic lymphadenectomy on patients with suspected lymph node metastases based on physical examination findings. This consists of excision of all lymph nodes in the affected regional lymph node basin.
Consider sentinel lymph node biopsy if no clinically positive nodes are present. With the use of blue dye, radioisotope, or both, injected at the site of the primary melanoma, the first-echelon node can be identified within the regional lymph node basin. Send this sentinel node to the pathologist for analysis using routine stains, immunohistochemistry, and even polymerase chain reaction in some centers. If the sentinel node is positive, then regional lymph node metastases is probable; importantly, perform a complete lymph node dissection. The correlation is based on the thickness of the primary tumor. If the sentinel lymph node is negative, the chance is 99% that all others are negative. This procedure is becoming the standard of care for tumors greater than 1 mm in depth.
Hyperthermic arterial limb perfusion with melphalan for extremity melanomas has been studied as an adjuvant therapy. One study found it to be beneficial in that it produced higher response rates and overall survival rates than those for surgery alone. Other studies do not demonstrate benefit.
Adjuvant chemotherapy and/or biological therapy are also undergoing clinical evaluation. One study demonstrated that high-dose interferon alfa-2b resulted in prolonged relapse-free survival and overall survival compared with no adjuvant therapy. A follow-up study by the same group demonstrated preliminary results indicating high-dose interferon achieved a relapse-free survival benefit over no adjuvant treatment but not over low-dose interferon. Neither high- nor low-dose interferon had a significant overall survival advantage compared with observation alone. High-dose interferon can be associated with significant toxic/adverse effects (ie, liver toxicity), and some patients require dose reduction because it may not be well tolerated.
Stage III
Wide local excision of the primary tumor with 2-cm margins remains first-line therapy. [1] No survival advantage is demonstrated with wider resection margins. Skin grafting or other tissue-transfer techniques may be necessary to close the defect. Perform regional lymph node dissection because a stage III melanoma represents nodal disease. If the nodal status is unknown, consider a sentinel lymph node biopsy to determine if the disease is stage I, II, or III. As in stage II disease, the treatment failure rate is higher with wide local excision alone in this group compared with stages 0 and I. Many clinical trials currently are exploring similar options as adjuvant therapy.
Stage IV
Advanced metastatic melanoma is usually refractory to standard therapy; thus, consider these patients for clinical trials. Some treatments have yielded various objective responses, although they are usually short-lived. Dacarbazine (DTIC) and the nitrosoureas, carmustine (BCNU) and lomustine (CCNU), produced a 20% objective response rate. Response rates for interferon alfa and interleukin 2 range from 8-22% and 10-20%, respectively. Studies have shown promising results. One study showed improved rates of overall and progression-free survival in patients with previously untreated metastatic melanoma with the BRAF V600E mutation who received vemurafenib versus standard dacarbazine. [36] Another trial showed improved survival for metastatic melanoma patients treated with ipilimumab and dacarbazine versus placebo and dacarbazine. [37]
Another study looking at treatment of advanced extremity melanoma showed that hyperthermic isolated limb perfusion therapy is a more effective way of controlling this type of situation than isolated limb infusion therapy. [38] Currently, other studies in progress are comparing other cytotoxic and biologic drug regimens.
Surgical resection of isolated metastases in the gastrointestinal tract, the brain, the lungs, or bone may be performed for palliation, with occasional long survival. Metastatic lymph nodes also may be removed for palliation. Radiation may provide symptomatic relief for metastases to bone, the brain, or viscera. In-transit metastases arise in the lymphatics or soft tissue between the primary lesion and the regional lymph node basin at a rate of 2-3%. In addition to wide surgical excision as for a primary lesion, isolated hyperthermic limb perfusion is probably the most effective treatment for extremity lesions. Radiation and intralesional BCG vaccine injections have had varied success.
Recurrent melanoma
Surgical excision offers the most efficacious results in sites where it can be accomplished; however, melanoma is usually refractory to most standard systemic therapy.
Preoperative Details
Evaluate for metastases. Begin this evaluation with a detailed history and physical examination. The most common metastatic sites, in descending order, are the regional lymph nodes, skin and subcutaneous tissue (in-transit lesions), and lungs. Other possibilities include the brain, the liver, and bone.
In the absence of signs or symptoms of metastatic disease after performing the history and physical examination, obtain a chest radiograph. If abnormalities are observed, follow this with a chest CT scan.
Check serum alkaline phosphatase and lactate dehydrogenase levels. If these are abnormal or if the patient has a history of weight loss, anorexia, or abdominal pain, obtain a liver ultrasound, abdominal CT scan, or both.
Any abnormalities in the neurologic examination or a history of headaches, vertigo, numbness, or weakness warrant a head CT scan.
A bone scan also may be indicated for isolated bone pain or an elevated alkaline phosphatase level.
Use lymphoscintigraphy to identify the correct lymph node basin(s) that drain the site of the primary tumor. This is an essential aid in preoperative planning.
Intraoperative Details
When performing the wide local excision, first consider the surgical margins. If primary closure is not feasible, skin grafting or tissue transfers may be needed. The use of blue dye and radiotracer together provides excellent results and is sensitive in finding the sentinel lymph node. Frozen section specimens have largely been abandoned because the process destroys the ability to analyze the node using immunohistochemistry.
Postoperative Details
Send the lesion and lymph node specimens to experienced pathologists for review. After the primary tumor is evaluated, the lymph node(s) should undergo hematoxylin and eosin staining. If findings are negative for melanoma, use immunohistochemical techniques. Specific staining for S-100 protein and HMB-45 monoclonal antibody increases the detection of lymph node micrometastases. More elaborate techniques using reverse transcriptase polymerase chain reaction are still investigational. They can detect the tyrosinase gene, which is specific for the melanocyte.
Follow-up care
Close follow-up care is essential to monitor for new primary lesions, recurrence, or metastases. History and physical examination are the cornerstones, with further testing (eg, chest radiography, blood chemistries) if suggestive findings are encountered. Most practitioners observe patients every 3-6 months initially and then eventually decrease the frequency, although no consensus exists. Physicians observe patients with thicker tumors more frequently than patients with thinner lesions. Follow-up care with a dermatologist is strongly recommended. Educate patients on self-examination for detection of new or recurrent lesions and for recognition of the signs and symptoms of metastatic disease because the risk of developing a second melanoma or having recurrence is well recognized.
Complications, Prognosis, and Outcome
Complications
Inability to close the excision site primarily is not truly a complication; however, wound dehiscence, skin necrosis, or both can occur from closure under tension. Wound infection is a potential complication. Seromas and lymphoceles occur up to 27% of the time with axillary node dissection, while nerve dysfunction and/or pain can occur 22% of the time, and hematoma can occur 1% of the time. Lymphedema of the upper and lower extremities can complicate axillary and groin lymph node dissections. The rates vary from 2-39%. Avoid incomplete resection at all costs. Careful preoperative evaluation and meticulous attention to detail intraoperatively should help minimize the risk of these complications.
Poor prognostic factors include the following:
-
Tumor thickness (worse prognosis in thicker lesions)
-
Evidence of tumor in regional lymph nodes (stage III disease)
-
Higher number of positive lymph nodes
-
Presence of distant metastasis (stage IV disease)
-
Anatomic site (trunk and/or face lesions have worse prognoses than extremity lesions)
-
Presence of ulceration
-
Presence of regression on histologic examination (controversial)
-
Male sex
Outcome
The 5-year survival rate refers to the percentage of patients who live at least 5 years after their cancer is diagnosed.
Relative survival rates take into consideration the fact that people may die of other causes besides melanoma. With relative rates, anyone who died of another cause, such as heart disease, is not counted. This is considered to be a more accurate way to describe the prognosis for people with particular types and stages of cancer. Of course, 5-year survival rates are based on patients diagnosed and initially treated more than 5 years ago. Improvements in treatment often result in a more favorable outlook for recently diagnosed patients.
-
Stage 0: The 5-year relative survival rate is 97%.
-
Stage I: The 5-year survival rate is 90-95%. If a sentinel node biopsy yields findings of melanoma in the lymph nodes, the 5-year survival is approximately 75%.
-
Stage IIA: The 5-year relative survival rate is approximately 85%. If a sentinel node biopsy yields findings of melanoma in the lymph nodes, the 5-year survival is approximately 65%.
-
Stage IIB: The 5-year relative survival rate is approximately 72-75%. If a sentinel node biopsy yields findings of melanoma in the lymph nodes, the 5-year survival is 50-60%.
-
Stage IIC: The 5-year relative survival rate is approximately 53%. If a sentinel node biopsy yields findings of melanoma in the lymph nodes, the 5-year survival is approximately 44%.
-
Stage III: The 5-year survival rate is approximately 45%. It is higher if the melanoma has spread to only one node and is lower if it has spread to more than 3. It is higher if the spread can only be seen under the microscope. It is lower if the melanoma was ulcerated.
-
Stage IV: The 5-year survival rate for stage IV melanoma is approximately 10%. It is higher if the spread was to skin or distant lymph nodes.
In a study from Alabama, patients with 1, 2-4, or more than 4 positive node(s) had survival rates of 58%, 27%, and 10%, respectively. Patients with spread to the lymph nodes have an 85% chance of developing occult disease. The worst outcome is predicted for patients with distant metastasis (stage IV). With a single metastatic site, the 1-year survival rate is 36%, but this drops to 13% with 2 sites. Patients with 3 or more sites of metastatic disease essentially have a 0% survival rate in the first year. These rates all vary somewhat according to the prognostic characteristics
A retrospective study by Tellez et al suggested that cutaneous melanoma is more dangerous when found in women who are pregnant or have given birth within the past year. The study found that 12.5% of women diagnosed during or within a year after pregnancy had posttreatment cancer recurrence and that 25% had metastasis, versus 1.4% and 12.7%, respectively, of other women in the report. (Follow-up was typically at least 7 years.) However, the investigators cautioned that because the medical center where the study was conducted tended to see more complex cases, the study results might not be universally illustrative. [39, 40] Nonetheless, a literature review by Kyrgidis et al indicated that mortality in women with pregnancy-associated melanoma is 17% greater than in females with melanoma that is unrelated to pregnancy. [41]
Downey et al found in a retrospective study of patients with melanoma who underwent regional lymphadenectomy that the presence of extranodal extension and the American Joint Committee on Cancer (AJCC) stage of the melanoma, but not therapeutic versus completion lymph node dissection, influenced the cancer’s regional recurrence. Multivariate analysis determined the hazard ratio (HR) to be 2.77 for extranodal extension and 2.51 for patients with an N3 versus an N1 AJCC lymph node stage. [42]
Future and Controversies
The continuation of clinical trials to search for beneficial chemotherapy, gene therapy, biologic therapy, immunotherapy, and other adjunctive procedures is necessary to improve the prognosis for patients with melanoma. This is especially important as the number of patients continues to rise. As with all disease, methods for early detection in addition to careful physical examination are of utmost concern. Continued searches for serum markers and genetic markers are also essential. Creation of a successful public awareness campaign, such as those in Australia and New Zealand, is crucial.
Questions & Answers
Overview
What is the prevalence of melanoma in the US?
What is the global prevalence of melanoma?
What is the pathophysiology of melanoma?
What are the signs and symptoms of melanoma?
How is melanoma characterized?
What are the types of melanoma?
What is the role of biopsy in the diagnosis of melanoma?
What are the surgical options for melanoma?
What anatomy is relevant to melanoma?
What is lentigo maligna melanoma?
What are the types of melanoma?
What is superficial spreading melanoma?
What is acral lentiginous melanoma?
What is desmoplastic melanoma?
How is melanoma classified and staged?
What is the Breslow classification of melanoma?
What is the Clark classification of melanoma?
What is the TNM (tumor, node, metastasis) staging system for melanoma?
What are the American Joint Committee on Cancer (AJCC) staging groups for melanoma?
What is the role of medical therapy in the treatment of melanoma?
What is the role of surgical therapy in the treatment of melanoma?
How is stage 0 of melanoma treated?
How is stage I of melanoma treated?
How is stage II of melanoma treated?
How is stage III of melanoma treated?
How is stage IV of melanoma treated?
What is the role of surgical excision in the treatment of recurrent melanoma?
What is included in preoperative care of patients with melanoma?
How is melanoma excision performed?
What is included in postoperative care following excision of melanoma?
What is included in long-term monitoring following treatment of melanoma?
What are complications of melanoma?
Which factors predict a poor prognosis for melanoma?
What are the survival rates for melanoma?
Which treatments for melanoma are under investigation?
-
Malignant melanoma. Courtesy of Hon Pak, MD.
-
A 1.5-cm melanoma with characteristic asymmetry, irregular borders, and color variation. Courtesy of Wendy Brick, MD.
-
Lentigo maligna melanoma, right lower cheek. Centrally located erythematous papule represents invasive melanoma with surrounding macular lentigo maligna (melanoma in situ). Courtesy of Susan Swetter, MD.