History of the Procedure
Hippocrates provided one the first known references that stressed the importance of nerves, warning physicians to avoid injuring the nerves within the dislocated shoulders of soldiers during repair. Sushruta, through the observation of different injuries and their results, was able to describe the function of many components of the peripheral nervous system. Galen differentiated nerves from tendons and reported the successful repair of nerves by other physicians, although no record exists that he attempted any repairs (130-200 AD). In the seventh century, Paulus Aegineta was the first to report the use of suture and agglutination to repair nerves. Other physicians who performed early work with nerves and their repair include Rhazes and Avicenna in the ninth century, Ali Abu Ibn Sina in Persia during the 10th century, and Ferrara in Italy in the 17th century. [1, 2, 3, 4, 5]
Despite this early record of physicians attempting to classify and treat nerve injuries, the common belief prior to the 19th century was that nerves did not regenerate. As a result, any kind of major nerve injury was treated nonsurgically or with amputation. During the 19th century, the development of improved microscopic devices, along with enhanced staining techniques, allowed a more detailed examination of nerves and nerve tissue. These new techniques allowed Waller to describe what happens to a nerve once transected, Cruikshank to note the regrowth of nerves, and Cajal (1905) to clarify the events and manner of axon regeneration. These discoveries have since been refined and now form the basis for the current model of axon elongation following injury. [6]
Not until the 20th century and the series of large wars that created many nerve injuries were the clinical and surgical techniques used to repair nerves today successfully refined and implemented. Mitchell, who practiced during the US Civil War, found that nerve injuries were often associated with a burning pain (ie, causalgia) in the distribution of the affected nerve. [7] Tinel characterized a tingling sensation that occurs with regenerating nerves from his work with patients during World War I.
During World War II, Seddon [8] and Woodhall, working in different countries, refined several methods of nerve closure using cable grafts, bridging grafts, and primary and secondary closure. Soon after World War II, Sunderland published his findings on the topographic organization of nerves, which resulted in new techniques regarding the repair of fascicles. Most recently, Millesi [9, 10] pioneered the incorporation of microsurgical instruments and technique, which greatly have improved the results of nerve repair. [11, 4]
Problem
Nerve injury can be defined as a defect that results in a disruption of a nerve such that it can no longer transmit an action potential. A wide range of injury types and severities have been classified and should be considered. Two classification schemes have been practiced widely by clinicians to describe nerve injuries (see Table 1). The scheme proposed by Seddon [8] in 1943 uses the terms neurapraxia, axonotmesis, and neurotmesis to describe the severity. The second scheme, proposed by Sunderland, divides the gamut of injury types into 5 degrees that overlap nicely with Seddon's but provide a more specific scale for grading patients. [8, 1, 1, 12, 4, 13, 14]
Table 1. Clinical Progression Based on Degree of Injury (Open Table in a new window)
Degree |
Severity |
Description |
Tinel Sign |
Progress Distally |
Recovery Pattern |
Rate of Recovery |
Surgery |
First |
Neurapraxia |
Demyelination with restoration in weeks |
— |
Fast |
Complete |
Fast (days to 12 wk) |
None |
Second |
Axonotmesis |
Disruption of axon with regeneration and full recovery |
+ |
+ |
Complete |
Slow (3 cm/mo) |
None |
Third |
|
Disruption of axon and endoneurium causing disorganized regeneration |
+ |
+ |
Varies* |
Slow (3 cm/mo) |
Varies |
Fourth |
|
Disruption of axon, endoneurium, and perineurium, with intact epineurium and no regeneration |
+ |
— |
None |
None |
Yes |
Fifth |
Neurotmesis |
Transection of the nerve |
+ |
— |
None |
None |
Yes |
Sixth |
Neuroma-in-continuity |
Mixture of one or more of the above conditions |
Varies by fascicle, depending on injury |
||||
*Recovery is at least as good as nerve repair but varies from excellent to poor, depending on the degree of endoneurial scarring and the amount of sensory and motor axonal misdirection within the injured fascicle. |
|||||||
Neurapraxia (nerve nonfunction), the mildest form of injury, corresponds to a first-degree injury and usually involves demyelination without axon disruption and degeneration. Transient loss of function, or conduction block, results until remyelination occurs. This type of injury has a relatively short recovery time, and full function is expected without intervention by 12 weeks after presentation. [4] Because axons are myelinated to a different extent, function is lost and regained at different times. Motor fibers are the first lost and the last regained, while pain and sympathetic fibers are the opposite. [15] Common examples of this kind of injury include pressure palsies such as those that result from a tourniquet or from sleeping with pressure on a nerve, eg, Saturday night palsy. [4]
Axonotmesis (axon cutting), which occasionally is grouped with neurapraxia, describes the situation when axons, myelin, and associated internal nerve structures are disrupted. This overlaps with second-, third-, and fourth-degree injuries. The characteristic nerve changes that occur with these injuries involve the internal structures, while the external structures remain intact. This means that axons are disrupted and must regenerate, while the epineurium is intact and the nerve looks normal upon macroscopic examination. These injuries often result from situations in which traction overcomes the inelastic internal structures but leaves the elastic epineurium intact. [1, 15, 4, 14]
Second-degree injuries are those in which only the axons of a nerve are disrupted, leaving the endoneurium and the rest of the nerve intact. Because the axon is cut, degeneration and regeneration occur. This is the first stage of injury that shows an advancing Tinel sign. Because the endoneurium is intact, little scar tissue is expected. The nerve should fully regenerate, with complete sensory and motor function regained. Timing varies based on the location of damage relative to the end organs. [4]
Damage to the axons and the endoneurium that leaves the perineurium and epineurium intact is known as third-degree injury. This leaves the blood-nerve barrier intact but provides a disorganized bed through which the axons can travel. This can result in axon misdirection and potentially debilitating consequences. Fortunately, nerve organization is maintained on a large scale with fascicular continuity. Nerve regeneration may be slowed due to the infiltration of scar tissue or a smaller number of axons capable of survival and regeneration. An advancing Tinel sign, although somewhat slowed, should be present. The final result is not likely to be perfect; however, surgery is not normally indicated because targeting from the axon to the end organ is still relatively intact. [1, 12, 4, 14]
The worst form of closed nerve damage, fourth-degree injury, involves damage to all structures except the epineurial covering. Disruption of the perineurium results in a failure of the blood-nerve barrier and electrolyte regulation within the nerve space. This disables the intact axons that are still present so that no conduction down the nerve is possible. Axon regeneration is disorganized, and surgical intervention is required to restore function, although this may not be recognized immediately. At this stage of injury, a Tinel sign is present at the level of injury and does not move distally because the regenerating nerve is kept from advancing by large amounts of scar tissue or debris.
These injuries usually result from very severe stretch or traction injuries. Because the method of injury is similar to second- and third-degree injury and the nerve is continuous, waiting several weeks before surgical intervention is recommended because less serious injuries usually show improvement within the first 3 months. [1, 12, 4, 14]
Neurotmesis (nerve cutting) is the most severe type of injury and corresponds to a fifth-degree injury. This kind of injury results from a disruption in the continuity of the axons and all supporting structures, including the epineurium. This division involves a separation of the nerve ends such that axon regeneration from the proximal end is unlikely to reach the distal end. This class of injury is easy to diagnose because it usually involves an open wound with nerve deficits. Surgical repair is required for any return of function. The time required for return of function after repair depends on the location of the lesion and other variables. One must understand that nerves regenerate slowly, progressing 1-1.5 mm/d or 1 in/mo. Prognosis varies greatly depending on the circumstances but, in general, is guarded. [1, 12, 4, 14]
More recently, Mackinnon [4] described a sixth degree of injury. This new class was characterized to describe lesions with a mixture of the above findings, which occur fairly often and otherwise are known as neuromas-in-continuity. This type of injury has a mixed pattern of regeneration with complete recovery of first- and second-degree injuries and failure of recovery in areas with fourth- and fifth-degree injuries. This makes repair difficult because some fibers within a nerve are intact and should not be disrupted, while others are disrupted and have formed neuromas that must be dissected and repaired. The author feels that the microneurodissection involved with repair of this type of injury is one of the most challenging parts of nerve repair. Once the scar and neuroma(s) have been removed, care should be taken to reattach formerly continuous fascicles. [1, 12, 4, 14]
Epidemiology
Frequency
Nerve injuries are involved in a significant number of admissions to hospitals. While most of these injuries involve nerves within the head and spine, 5-10% of patients are found to have a peripheral neuropathy either in isolation or in combination with other injuries. [16] These injuries occur more often in men than in women, with a male-to-female ratio of 2.2:1. They also occur more commonly in the dominant hand, most likely as a result of reflexive protective motions. [17, 13] When researchers looked at the individual nerves of the arm, they found that the digital nerves were damaged most frequently, followed by the median nerve, the ulnar nerve, and, finally, the radial nerve. The most commonly injured digital nerves were in the ulnar and radial distributions. [5] For more information, see the Medscape Drugs & Diseases article Nerve Compression Syndromes of the Hand.
Etiology
While many possible causes exist for nerve injury, some of the more common involve falls, collisions, motor vehicle accidents, high-velocity missiles, fractures, dislocations, lacerations, or some other form of penetrating trauma. These causes may be grouped into general categories including penetrating injury, which generally involves sharp transection; trauma-type injury, which generally involves some kind of crush component; massive tissue loss; and avulsion or traction injuries, which result in stretching or tearing of the nerve internally due to an excessive amount of tension.
Ischemia and other nonmechanical factors, such as thermal injury, electric shock, radiation, percussion, and even envenomation, also can cause substantial nerve damage. Because of the difficulty in diagnosing a nerve injury in the presence of polytrauma, care must be taken to perform a thorough examination for sensory and motor loss as soon as possible. [18, 13]
Pathophysiology
All nerve injury results in a predictable response. While all the changes that occur after a nerve injury are usually considered a normal physiologic response, it is a normal response to a very abnormal situation. Depending on the type of injury, the response is different but still somewhat predictable. If the axon is spared, such as in first-degree injury, conduction is interrupted due to demyelination, but it is reinstated whenever the aggravating stimulus is removed and the myelin layers are restored. If the axon, or more, is transected, causing a second- to fifth-degree injury, the response has 2 main phases, degeneration and regeneration, and takes substantially longer.
Table 2. Pathophysiology of Degeneration and Regeneration* (Open Table in a new window)
Timing |
Degeneration |
Regeneration |
6 hours |
Nucleus becomes displaced and Nissl bodies break up, turning the cell basophilic. [15] |
Axon spikes appear briefly at the proximal end. |
1 day |
Macrophages begin entering the site of degeneration. This stimulates Schwann cell proliferation [19] Nerve function drops off with rupture of the blood-nerve barrier. [20] Distal stump begins to swell. |
Growth cones that contain a cytoskeleton form at the end of axon sprouts. Cell bodies of severed axons begin to enlarge as the cells become activated. The nucleus must become hypochromatic before elongation can occur. [21] |
2 days |
|
Mitochondria in the axoplasm for distal transport. |
3 days |
Degenerative process involves all myelinated axons. Perineurial cells become enlarged and active. Axons shrink, and myelin begins to disintegrate. This is cleaned up by macrophages and Schwann cells and can take as many as 3 months. [14] Schwann cell proliferation peaks. [19] |
|
4 days |
|
RNA production increases in the cell body. Axon sprouting may begin at day 4 in a clean transection. [21] |
1 week |
Infiltration of inflammatory cells and RBCs occurs, along with myelin fragmentation. |
Schwann cells are activated and dividing. Growth cones can occasionally be seen within a Schwann cell, depending on the injury type. Swelling of axoplasm occurs in myelinated fibers, caused by mitochondria. |
2 weeks |
Schwann cell proliferation has peaked, and endoneurial clearance is proceeding. As the contents of the tubes are removed, they shrink; if collagen is laid down, the reduced size can become permanent. [19, 22, 23] |
Schwann cells near regenerating axons stop myelin destruction and surround axons. [24] |
3 weeks |
The distal portion of the axon is finishing the degenerative processes, and the myelin is fragmenting. [21] |
The axon is surrounded completely by myelin, and the organelle count in the Schwann cell drops. Most of the regenerating axons are found outside the degenerating endoneurial tubes. [24] Metabolic changes in the axon peak. [20] Axon sprouting usually starts and can cross the anastomoses. [21] |
4 weeks |
|
Remyelination starts, and perineurial cells decrease in size once the nerve is remyelinated. |
*Times can vary extensively with the type and extent of damage. [25, 24, 14] |
||
The first phase of axon injury, degeneration, has been known as wallerian degeneration since Waller's description in 1850. Following injury, changes occur in the axon, cell body, and the Schwann cell covering of the nerve to help prepare for regeneration. Distally, the axon begins to disintegrate and undergoes apoptosis, releasing vesicles of cytosol and organelles. This same process occurs proximally, although the extent varies with the severity of injury and is eventually stopped by the regeneration process. Local Schwann cells and macrophages that migrate across the local vessels clean up the apoptotic debris, creating long, clean endoneurial tubes.
Once the debris has been removed and the macrophages have returned to the peripheral circulation, Schwann cells begin to proliferate and organize themselves into columns that lie within the endoneurial tubes, thus creating what are known as the bands of Bunger. [1, 25, 21, 26, 24, 4, 27, 28, 29, 30, 31, 14]
While the distal portion of the nerve is being cleared and prepared for reinnervation, the proximal portions of the neurons are going through a process of regeneration. Immediately after injury, the cell body swells, the nucleus becomes hypochromatic, and the production of mRNA and proteins is greatly increased. [1, 21, 27] These products are transported down the axon, providing the material and energy for nerve elongation to the distal tip. While the cell body is busy making raw materials, the newly severed axon end begins to sprout, initially sending out transient axonal processes that are retracted and replaced by more permanent filopodia that contain a cytoskeleton and are capped by an expanded region known as the growth cone.
The growth cone has been shown to be the site of axon elongation and is the location of selectivity. This structure sends out many small processes that seek specific markers, which influence the axon in its movements to preferentially select neural tissue and even exhibit a preference for endoneurial tubes that have the same function. For instance, when a motor axon reaches a nerve gap, it moves across small gaps into the severed distal end and finds a motor tube to begin growing down. This helps preserve function but is not specific enough to recreate innervations identical to those that existed prior to injury. [32, 33, 34, 24, 4, 19, 35] Forssman first described this mechanism in 1898, and it has been elucidated further by Cajal, Weir, Weis, and many others.
The axon's response is known to be regulated by chemical signals that exhibit neurotropic and neurotrophic influences. Neurotropic factors are responsible for directing and attracting the regenerating axons. Neurotrophic factors are involved with inducing maturation and elongation of the axon. [36, 37, 38, 29, 39] Macrophages, Schwann cells, and other supporting cells in the area release these chemical factors and attempt to create a microenvironment that promotes axon elongation. Limits exist on directing axon regeneration. If the nerve is too far away, the axons are not strongly attracted to the distal end and eventually stop advancing, resulting in a neuroma. If the nerve is too close, the axons cannot exhibit specificity of function, causing severely aberrant patterns of innervation at the end organ level. [21, 24, 40, 20, 41, 28, 29, 31]
Despite the systems in place for proper reinstatement of nerve function, many different events can impede axon sprouts. Depending on the severity of the injury, the proximal axon stump may have retracted. Usually, more retraction occurs with more severe injuries, and the regenerating axon requires longer to reach the nerve gap. Additionally, the possibility exists that the metabolic demands placed on the cell for regeneration exceed the capabilities of the cell, eventually leading to atrophy.
Large gaps, usually those greater than 15-30 mm, cannot be crossed reliably by axons. This is usually because proliferating Schwann cells or fibroblasts grow between the severed nerve ends and form a physical blockade. [42] Alternatively, if suitable tissue is not found within a set distance, the axon sprouts stop proliferating and take residence in nonneural tissue, forming a neuroma. Neuromas can occur in any situation in which an axon or collateral sprout remains in nonneural tissue. [40, 4, 27, 43, 39]
Presentation
To accurately diagnose a nerve injury, obtain a thorough history from the patient. The history should include the patient's age, occupation, avocations, hand dominance, and previous injuries and procedures. Additional information that can be helpful includes allergens, medications, past medical history, and the events leading to the injury. In the acute setting, a nerve injury should be expected and sought whenever a patient presents with an open cut or laceration. Also, look for symmetric movement of the limbs because a lack of movement is suggestive of damage. [39]
The physical examination should focus on determining the level of nerve injury and should attempt to differentiate partial from complete lesions. This can be difficult if the patient is not fully coherent. The examination should include a motor evaluation and a sensory evaluation. These tests must be performed in a very detailed manner so that accurate data are found to aid with diagnosis. [6, 44, 45, 39, 46]
The motor evaluation should include tests to determine the range of motion, functionality, and strength in the muscles supplied by the nerve. Each nerve should be tested individually using isolated movements as much as possible. The radial nerve is tested using extension of the elbow, wrist, and fingers. The median nerve innervates both extrinsic and intrinsic muscles of the hand. Extrinsic median motor function can be tested with flexion of the index finger at the distal and proximal interphalangeal joints, thumb flexion on the interphalangeal joints, and radial wrist flexion.
The intrinsic median innervation of the hand can be tested with thumb abduction. The ulnar nerve also has both extrinsic and intrinsic components. The extrinsic ulnar musculature can be tested using proximal interphalangeal flexion of the small finger and ulnar wrist flexion. The intrinsic muscles of the hand, the interossei, are used to test intrinsic innervation. All movements can be compared bilaterally for strength and range of motion. Functionality of muscle innervation can be tested using complex motions such as twisting or manipulating various objects.
The sensory evaluation is used to test for basic protective sensation and 2-point discrimination (2PD) and to map out all areas of paresthesia. The radial nerve supplies the dorsal radial aspect of the hand and the first web space. The median nerve supplies the index finger, thumb, and proximal palm near the thenar eminence by way of the palmar cutaneous branch. The ulnar nerve supplies the ulnar side of the hand and little finger. It also supplies the dorsal ulnar region of hand via the dorsal cutaneous branch of the ulnar nerve. The digital nerves lie adjacent to the distal interphalangeal flexion crease and supply the adjacent areas, but not the fingertips.
Adjuvant findings that can help with localizing innervation deficits are the presence of dry, shiny skin and the presence of skin that does not wrinkle when immersed in water. These findings are the consequence of denervation of the skin. Also, a Tinel test can be used to locate the ends of transected and regenerating axons. If the findings remain ambiguous or unclear after the examination, the patient should be sent for electrodiagnostic procedures so that the extent and grade of the injury can be determined. [19, 46]
Indications
Determining the optimal treatment for a patient is a complex task that depends on the results of a complete patient assessment. The questions involved with nerve repair involve 2 main areas, namely, (1) when to do the repair and (2) what type of repair to perform. Primary repair is generally considered within the first few days, delayed primary by the end of the first week, and secondary closure beyond that. [19, 5, 14] Surgical options generally include end-to-end closure, nerve graft placement, vascularized nerve graft placement, and nerve transfer.
Table 3. Selection of Operative Procedure (Open Table in a new window)
Surgery |
Ends Can Approximate |
Vascularized Bed |
Graft Possible |
Proximal Portion Intact |
Distal Portion Intact |
End-to-end closure |
Yes |
Yes |
Yes |
Yes |
Yes |
Nerve graft |
No |
Yes |
Yes |
Yes |
Yes |
Vascularized graft |
No |
No |
Yes |
Yes |
Yes |
Conduit |
No |
No |
No |
Yes |
Yes |
Nerve transfer |
No |
No |
No |
No |
Yes |
Primary closure has been found to be superior to secondary closure. [35, 18] However, to ensure that the repair is successful and undisturbed during the healing process, certain criteria should be met. The condition of the wound itself is important. It should be a limited lesion, preferably a sharp transection, rather than a crush-type injury. If a crush component is present, it should be limited enough that coaptation of nerve ends is still possible after resection of damaged tissue. The wound should be free of debris and contamination that may compromise healing (eg, from infection). Considering whether the wound provides an adequately vascularized bed for the nerve is also advisable because this aids the healing process.
Supporters of delayed repair suggest that waiting provides increased fibrosis and mechanical support to the wound and suture. Some also suggest that waiting to perform the repair allows it to coincide with maximal axoplasmic synthesis, which occurs approximately 3 weeks after injury, although this has not been demonstrated to be beneficial in most patients. [19, 14]
Another consideration is the condition of the patient. Large numbers of concomitant injuries, especially in the same area, present contraindications because of the stress on the patient and the mechanical disruption that can occur during additional repair surgeries. This means that in a complex wound, the nerve repair should be delayed until all other injuries have been treated. The patient should be stable enough to be considered for elective surgery, and the patient should be able to give informed consent. [19, 14]
The final part of the equation concerns the facilities and staff available at the time. In order to maximize the repair effort, microsurgical equipment should be available and the primary surgeon should be familiar and comfortable with this type of surgery. [14, 9]
As long as the above conditions are met and the defect is repaired in a tensionless manner, the result should be favorable. If any of the criteria are not met, waiting and performing a delayed primary or secondary repair of the nerve is better.
Surgically, the most favorable form of repair is the end-to-end approximation. This type of surgery is indicated in sharp transections, in which the ends of the nerve can be found, are relatively intact, and are close enough to be approximated without tension. If the injury is proximal, fascicular repair more fully restores the disrupted pathways. Distally, where the axons are grouped more or less by function and the nerves are much smaller, endoneurial repair can suffice. [19, 14]
In situations in which the wound is more complex, such as with a crushing trauma, burn injury, or failed end-to-end closure that needs resection and repair, alternative methods may be advised. Crush wounds generally create a fairly large nerve gap that cannot be closed without placing the coaptation under significant tension. Some have suggested that in the upper arm, a nerve gap of 4-5 cm [4, 19] requires closure by grafting.
In the small digital nerves, this is reduced to gaps greater than 1 cm, which can usually be closed with finger positioning. Whenever the wound bed is well vascularized and free of contamination, a conventional nerve graft can be performed. [9] In situations that are more rigorous, such as a burn, a vascularized nerve graft can help ensure the survival of the graft and may hasten regeneration. [47, 40, 48] For this reason, vascularized grafts also may be beneficial in proximal injuries when regeneration is likely to take a long time. Despite the increased regeneration speed and graft survival, no significant difference is found in the resulting function or sensation when compared to a normal graft procedure.
Conduits and nerve transfers are the final 2 surgery types, but they are performed only under very specific situations. Conduits are indicated in patients in whom a small nerve gap is present but no autologous nerve is available for transfer (or not enough to completely repair the defect). Instead, conduits can be made out of blood vessels, muscle, and occasionally a synthetic material such as silicon or polyglycolic acid. [42, 49]
Nerve transfers are rarely performed and are reserved for situations of massive injury when other forms of nerve repair are unavailable and tendon transfers are exhausted or impossible. This technique uses the proximal portion of an intact nerve to reinnervate the distal portion of a more important nerve once the proximal portion has been destroyed. [50] This also may be necessary when addressing a delayed presentation of injury or a lesion that is older than 6 months.
Relevant Anatomy
Nerve anatomy
Peripheral nerves, such as in the CNS, are arranged to provide an isolated and protected region within which neurons operate. The functional parts of neurons contained within the peripheral nerve are the long processes, axons, and modified dendrites that communicate with distant end organs. These long processes, in the case of axons, carry messages from the anterior horn cells in the spinal cord to effectors and, in the case of dendrites, carry messages to dorsal root ganglia from various sense organs. The axons are surrounded by Schwann cells, which provide nutrition, support, and protection.
Axons can be myelinated, with Schwann cells wrapped around a single axon, or unmyelinated, in which case the Schwann cells cover many different axons without creating the myelin layers. Around each axon and its Schwann cells is a layer of loose connective tissue and fluid that comprises the endoneurium. This combines with the basement membrane of the Schwann cell to create the endoneurial tubes that surround the axons. [4, 51, 52, 39, 31, 14, 53]
The next layer of organization arranges axons into bundles, or fascicles, that travel together. The fascicle number is not constant and can vary extensively through the course of the nerve. Another layer of connective tissue covers each fascicle. This layer is a continuation of the pia-arachnoid layer of the CNS and is one component of the blood-nerve barrier. This layer is made and maintained by concentric lamella of perineurial cells. The perineurial layer is very important to nerve function and must remain intact for the nerve to function. [54, 52, 39, 31, 14, 53]
Above the perineurium lies the epineurium. Inner and outer epineurial layers are present, with loose connective tissue and collagen between them. The inner epineurium is relatively vascular and surrounds individual and bundled fascicles. The thickness of the epineurium varies by location but can comprise 25-75% of nerve cross-section. [55] This allows the epineurium to provide some amount of protective cushioning to the inner structures. Also, the epineurium contains elastin that makes this outer cover slightly elastic. This property facilitates the longitudinal excursion and gliding movements of the nerve.
During rest, the epineurium contracts, creating striations within the nerve termed the bands of Fontana. These disappear with excursion as the epineurium stretches and allows the nerve to extend to full length. Problems arise when the epineurium contracts after nerve transection, causing the ends to retract and the nerve gap to enlarge. Also, the elastic properties of a nerve can be altered greatly by surgery because of adhesions that form around the area of repair. This alters the amount of excursion available and causes the free parts of the nerve to stretch more than normal. The epineurium is primarily a protective layer and, unlike the perineurium, can be removed without detriment to nerve function. [52, 39, 31, 14, 53]
Vasculature and innervation
Neural tissue requires a significant amount of oxygen to function properly, and, as a result, peripheral nerves have an extensive blood supply composed of both external and internal blood vessels. The external vessels run through the perineurium and segmentally supply the intrinsic vessels via the arteriae nervorum. The intrinsic vessels are arranged in a longitudinal plexus made from the vasa nervorum that run through the outer epineurium, inner epineurium, and perineurium. A series of endoneurial vessels that supply the axons themselves is also present. These endoneurial capillaries have epithelial tight junctions that function as the second part of the blood-nerve barrier.
The longitudinal arrangement of the vessels within the nerve may allow the surgeon to perform a fair bit of dissection along the course of the nerve without compromising vascular integrity to the point of ischemia. However, care must be taken to not injure any major nutrient vessels that are associated with the nerve. [54, 39, 31, 46]
Topography
Initially, when Sunderland looked at the fascicular patterns of peripheral nerves in 1947, [56] he described a pattern of twisting and crossing fascicles that branched so often that nerve grafting and intraneural dissection were assumed to be impossible. Fortunately, the pattern described by Sunderland is only true for the proximal part of the nerve. As described by Jabaley in 1980, [51] the fascicular pattern in the distal forearm is much straighter, with less crossing over. Apparently, the crossing over in the proximal portion sorts the nerve fibers into bundles by function. This means that by the time the distal forearm is reached, the fascicles contain nearly pure motor or sensory axons. [4, 14] While the arrangement varies by individual and by nerve, generally, the sensory fascicles are considered to sit more superficially and the motor fibers more dorsal. [57, 9, 58]
Median nerve
The median nerve originates in the brachial plexus as branches from the lateral and medial cords come together. [59] These cords bring fibers from all roots of the brachial plexus, from C5 to T1. The median nerve runs through the anteromedial compartment, through the cubital fossa just medial to the brachial artery, and enters the forearm between the heads of the pronator teres. [4, 52] The median nerve supplies most of the flexor muscles in the forearm and a few muscles in the hand (see Motor targets of the median nerve). Once in the forearm, the median nerve splits into a superficial and deep branch. The superficial branch supplies the pronator teres, flexor carpi radialis, and palmaris longus before the distal portion supplies the flexor digitorum superficialis.
The deep trunk runs medially down the forearm deep to the muscles and supplies motor innervation to the flexor pollicis longus, pronator quadratus, and the portions of flexor digitorum profundus (FDP) that flex the index and long fingers and sends articulate branches to the radial carpal joint via the anterior interosseous. At the wrist, the deep median branch sits between the palmaris longus and flexor pollicis longus, travels through the carpal tunnel with the flexor tendons, [60] and splits into 6 branches once clear of the flexor retinaculum. The recurrent branch of the median nerve innervates the palmaris brevis and the muscles of the thenar eminence, namely the abductor pollicis brevis, opponens pollicis, the first and second lumbricals, and the superficial head of the flexor pollicis brevis.
The remaining branches are sensory and include 3 common digital nerves to the second, third, and fourth digits and 2 proper digital nerves to the thumb. These digital nerves run along the lateral (radial) and medial (ulnar) sides of the fingers with the digital arteries. The median nerve has 2 other sensory branches not yet mentioned that supply the elbow and a palmar cutaneous branch that passes over the top of the flexor retinaculum to innervate the palm. [61, 62]
Motor targets of the median nerve are as follows:
-
Pronator teres
-
Flexor carpi radialis
-
Palmaris longus
-
Flexor digitorum superficialis
-
Flexor digitorum profundus
-
Flexor pollicis longus
-
Pronator quadratus
-
Palmaris brevis
-
Abductor pollicis brevis
-
Flexor pollicis brevis - Superficial head
-
Opponens pollicis
-
First and second lumbricals
Some notable variations in the pattern of innervation exist in the median distribution. In the forearm, the median nerve supplies approximately one half of the FDP muscle, sharing it with the ulnar nerve. However, in 50% of patients, the median and ulnar nerves overlap and the median nerve encroaches on the ulnar innervation to the FDP. However, in the hand, the ulnar nerve tends to extend across the palm and innervate a larger portion of the thenar eminence. [31]
Another clinically important variation is the presence of Martin-Gruber anastomoses in the forearm and Riche-Cannieu anastomoses in the palm. Martin-Gruber anastomoses are believed to be present in approximately 17% of patients, while Riche-Cannieu anastomoses may be present in as many as 70%. Because these anastomoses allow unique patterns of innervation, they can mask lesions by changing the symptoms present. [63, 31]
Ulnar nerve
The ulnar nerve arises from the medial cord and contains fibers from the C7, C8, and T1 roots. [59] It passes through the arm, behind the medial epicondyle, and into the flexor compartment (see Motor targets of the ulnar nerve). In the forearm, the ulnar nerve gives off motor branches to the flexor carpi ulnaris and the medial (ulnar) portion of FDP that supplies the ring and little fingers. [52] Usually, the ulnar nerve gives a small branch to the ulnar artery, known as the nerve of Henle, which is present in approximately 60% of people. The remaining 40% of people have a palmar cutaneous branch. [64] At the level of the wrist, the ulnar nerve passes through the Guyon canal, right next to the hook of the hamate.
In the hand, the ulnar nerve divides into a deep motor and a superficial sensory branch. The deep motor branch supplies all of the intrinsic muscles of the hand: the muscles of the hypothenar eminence, the interossei, the third and fourth lumbricals, adductor pollicis, and the deep head of the flexor pollicis brevis. The superficial sensory nerve supplies sensation to the little finger and the ulnar side of the ring finger. The ulnar nerve also supplies sensation to part of the dorsum of the hand via the dorsal sensory branch that wraps around to the dorsum near the level of the dorsal carpal ligament. This provides sensation in the same distribution as on the volar surface. [61, 31]
Motor targets of the ulnar nerve are as follows:
-
Flexor carpi ulnaris
-
Flexor digitorum profundus
-
Hypothenar muscles
-
All interossei [65]
-
Third and fourth lumbricals
-
Adductor pollicis
-
Flexor pollicis brevis - Deep head
Radial nerve
The radial nerve is a branch off the posterior cord and contains fibers from roots C7-T1. [52, 66] This nerve wraps around the humerus in the spiral groove as it passes through the upper arm, supplying motor innervation to all 3 heads of the triceps muscle, the anconeus, the brachioradialis, and a small part of the brachialis muscle before entering the cubital fossa lateral to the biceps tendon. In the forearm, the nerve supplies motor innervation to the extensor carpi radialis longus, extensor carpi radialis brevis, and the supinator before splitting into deep and superficial branches (see Motor targets of the radial nerve).
The deep branch provides motor innervation to the muscles of finger and thumb extension before becoming the posterior interosseous nerve and supplying sensation to the dorsal aspect of the carpal joints. The superficial sensory branch passes through the anatomic snuffbox on its way to supply sensation to the dorsum of the hand, thumb, and first 2.5 fingers. The radial nerve also provides sensation to a portion of the forearm via the posterior cutaneous nerve and to the elbow. [61, 31]
Motor targets of the radial nerve are as follows:
-
Triceps (long, medial, lateral)
-
Anconeus
-
Brachioradialis
-
Extensor carpi radialis longus
-
Extensor carpi radialis brevis
-
Supinator
-
Extensor digitorum communis
-
Extensor indicis proprius
-
Extensor digiti minimi quinti
-
Extensor carpi ulnaris
-
Abductor pollicis longus
-
Extensor pollicis longus
-
Extensor pollicis brevis
Contraindications
Nerve repair has been described in virtually all clinical settings. However, experience and common sense dictate the following list of contraindications:
-
Medically unstable patient from other injuries and/or illnesses
-
Presence of a grossly contaminated wound bed
-
Active soft tissue infection in the region of the nerve injury
-
Severely compromised nutrition
-
Patient unable and/or unwilling to comply with required activity restrictions
-
Patient with unrealistic expectations
-
Presence of underlying skeletal instability
-
Uncertain delineation of zone of injury (requires waiting period)
-
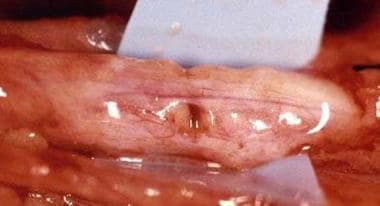
Hand nerve injury repair. Crushed median nerve at the elbow.
-
Hand nerve injury repair. Epineural repair of median nerve.
-
Hand nerve injury repair. Partial transection of ulnar nerve in the forearm.
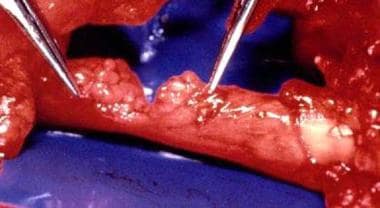
-
Hand nerve injury repair. Posttraumatic neuroma.
-
Hand nerve injury repair. Excision of posttraumatic neuroma.
-
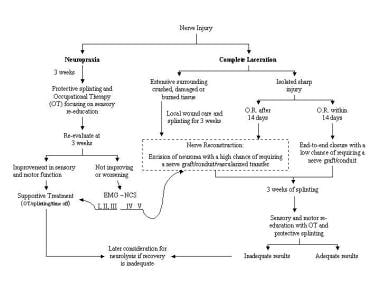
Hand nerve injury repair. Algorithm for the treatment of nerve injuries.