Classification and Principles of Flap Surgery
A flap is a unit of tissue that is transferred from one site of the body (donor site) to another (recipient site) while maintaining its own blood supply.
Flaps come in many different shapes and forms. They range from simple advancements of skin to composites of many different types of tissue. These composites need not consist only of soft tissue. They may include skin, muscle, bone, fat, or fascia.
How does a flap differ from a graft? A flap is transferred with its blood supply intact, and a graft is a transfer of tissue without its own blood supply. Therefore, survival of the graft depends entirely on the blood supply from the recipient site.
Flap surgery is a subspecialty of plastic and reconstructive surgery. Various types of flaps are performed, and the indications for them are even more diverse.
Rather than discuss all the different types of flaps ever used, this article aims to outline the broad classification of flaps and to provide basic principles to remember when preparing for the operating room. For more details on particular flap procedures, please find open access articles here.
Flap History and Classification
History of flap surgery
The term flap originated in the 16th century from the Dutch word flappe, meaning something that hung broad and loose, fastened only by one side. The history of flap surgery dates as far back as 600 BC, when Sushruta Samita described nasal reconstruction using a cheek flap. The origins of forehead rhinoplasty may be traced back to approximately 1440 AD in India. Some reports suggest flap surgeries were being performed before the birth of Christ.
The surgical procedures described during the early years involved the use of pivotal flaps, which transport skin to an adjacent area while rotating the skin about its pedicle (blood supply). The French were the first to describe advancement flaps, which transfer skin from an adjacent area without rotation. [1] Distant pedicle flaps, which transfer tissue to a remote site, also were reported in Italian literature during the Renaissance period.
Subsequent surgical flap evolution occurred in phases. During the First and Second World Wars, pedicled flaps were used extensively. The next period occurred in the 1950s and 1960s, when surgeons reported using axial pattern flaps (flaps with named blood supplies). In the 1970s, a distinction was made between axial and random flaps (unnamed blood supply) and muscle and musculocutaneous (muscle and skin) flaps. This was a breakthrough in the understanding of flap surgery that eventually led to the birth of free tissue transfer.
In the 1980s, the number of different tissue types used increased significantly with the development of fasciocutaneous (fascia and skin) flaps (which are less bulky than muscle flaps), osseous (bone) flaps, and osseocutaneous (bone and skin) flaps.
A further advancement in flap surgery came in the 1990s, with the introduction of perforator flaps. These flaps are supplied by small vessels (previously thought too small to sustain a flap) that typically arise from a named blood supply and penetrate muscle, muscle septae, or both to supply the overlying tissue. An example of this is the deep inferior epigastric perforator (DIEP) flap, which has now become the criterion standard in breast reconstruction. Noncontrast MRI is now used for preoperative mapping of perforator flaps. [2, 3]
Classification of flaps
Most classification systems have been designed for the sole purpose of aiding communication between peers by providing an appropriate vocabulary. However, the crucial point for any physician to remember is that communication with the patient is of foremost importance. The patient must be able to picture, with the surgeon's guidance, what the surgeon is planning.
Many different methods have been used to classify flaps. Furthermore, these classification systems are often complex and varied in principle.
To improve the reader's understanding of flap classification, the author has summarized the most commonly used classifications into three simplified categories: type of blood supply, type of tissue to be transferred, and location of donor site.
Blood supply
Like any living tissue, flaps must receive adequate blood flow to survive. A flap can maintain its blood supply in 2 main ways.
If the blood supply is not derived from a recognized artery but, rather, comes from many little unnamed vessels, the flap is referred to as a random flap. Many local cutaneous (skin) flaps fall into this category.
If the blood supply comes from a recognized artery or group of arteries, it is referred to as an axial flap. Most muscle flaps have axial blood supplies. Because of the complexity and variation observed in axial blood supply, a further subclassification (axial types I-V) was made by Mathes and Nahai and is readily used in plastic and reconstructive surgery literature to describe different types of muscle flaps (see the image below). [4]
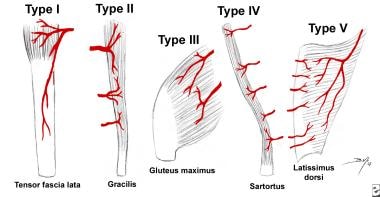 Patterns of muscle flap vascular anatomy. Type I - One vascular pedicle. Type II - Dominant pedicle(s) and minor pedicle(s). Type III - Two dominant pedicles. Type IV - Segmental vascular pedicles. Type V - One dominant pedicle and secondary segmental pedicles.
Patterns of muscle flap vascular anatomy. Type I - One vascular pedicle. Type II - Dominant pedicle(s) and minor pedicle(s). Type III - Two dominant pedicles. Type IV - Segmental vascular pedicles. Type V - One dominant pedicle and secondary segmental pedicles.
A perforator flap commonly derives its name not from its underlying muscle but from the main nutrient blood vessel. However, if, potentially, multiple perforator flaps can be harvested from one vessel, each flap is named according to the donor site’s anatomic region, or the underlying muscle. Potentially, any flap can be elevated as a perforator flap provided that there is complete preservation of its main nutrient blood vessel.
A literature review by Hamilton et al suggested that vascular delay (also known as ischemic preconditioning), in which rendering tissue partially ischemic encourages neovascularization, may permit complex flaps to be effectively used at sites with poor vascularization. [5]
Tissue to be transferred
In general, flaps may comprise in part or in whole almost any component of the human body, as long as an adequate blood supply to the flap can be ensured once the tissue has been transferred.
Flaps may be composed of just one type of tissue or several different types of tissue. Flaps composed of one type of tissue include skin (cutaneous), fascia, muscle, bone, and visceral (eg, colon, small intestine, omentum) flaps. Composite flaps include fasciocutaneous (eg, radial forearm flap), myocutaneous (eg, transverse rectus abdominis muscle [TRAM] flap), [6] osseocutaneous (eg, fibula flap), tendocutaneous (eg, dorsalis pedis flap), and sensory/innervated flaps (eg, dorsalis pedis flap with deep peroneal nerve).
Therefore, another way of classifying flaps is by describing the different types of tissue that are being used in the flap.
Location of donor site
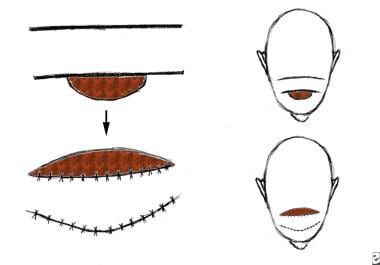
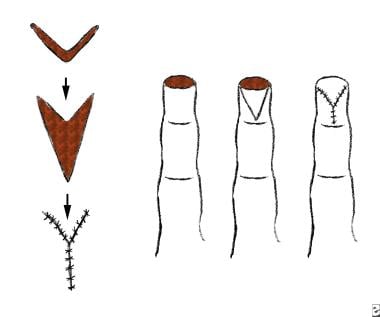
Tissue may be transferred from an area adjacent to the defect. This is known as a local flap. It may be described based on its geometric design, be advanced, or both. Pivotal (geometric) flaps include rotation, transposition, and interpolation. Advancement flaps include single pedicle, bipedicle, and V-Y flaps.
Tissue transferred from an noncontiguous anatomic site (ie, from a different part of the body) is referred to as a distant flap.
Distant flaps may be either pedicled (transferred while still attached to their original blood supply) or free. Free flaps are physically detached from their native blood supply and then reattached to vessels at the recipient site. This anastomosis typically is performed using a microscope, thus is known as a microsurgical anastomosis. Visible light spectroscopy can be used to measure tissue oxygenation in free flap reconstruction. [7, 8]
Principles of flap surgery
Now that the main ways of classifying flaps have been introduced, the remaining sections of this article are devoted to the most important principles to remember before performing flap surgery. Like any surgical procedure, flap surgery is not devoid of risk. Complications such as complete flap loss can be catastrophic. Considering the following basic principles before any flap surgery serves patients well by optimizing outcome and decreasing operative morbidity.
Principle I: Replace Like With Like
This is a particularly important principle. When filling in a defect, replace like with like. Ralph Millard once said, "when a part of one's person is lost, it should be replaced in kind, bone for bone, muscle for muscle, hairless skin for hairless skin, an eye for an eye, a tooth for a tooth."
If this cannot be accomplished, use the next most similar tissue substitute. For example, the surgeon can use scalp to replace a beard or skin from the forehead to cover a nose wound. The goal is to camouflage the reconstruction as much as possible. Everyone can learn from Mother Nature's blending tricks. The surgeon's goal is to create an effect as subtle as a chameleon changing colors as it moves through its surroundings.
An example of this can be found in the treatment of any eyelid injury. The best course of action when faced with a full-thickness defect is to use eyelid skin from the contralateral eye. If this is not possible, the next best substitute is a full-thickness posterior auricular skin graft. This provides the most similar substitute tissue, with a satisfactory color match and minimal tendency toward contracture.
If the surgeon's work can pass unnoticed, he is to be congratulated as having accomplished his task as a reconstructive surgeon.
Principle II: Think of Reconstruction in Terms of Units
According to Millard, human beings may be divided into 7 main parts: the head, neck, body, and extremities. Each of these body parts can be further subdivided into units. The head, for example, is composed of several regional units: scalp, face, and ears. Consider that each of these units has its own unique features, and each feature has, in turn, multiple subunits with their own special shapes. All of these different units and subunits must be considered and reproduced during reconstruction.
As emphasized by Millard, "The most important aspects of a regional unit are its borders, which are demarcated by creases, margins, angles and hair liners." Taking this a step further, perhaps the most important principle is the way in which the borders between units come together and interact rather than just the borders themselves.
Adherence to these natural borders during reconstruction is important. Most often, it is better to convert a defect that covers only a partial unit to a whole-unit defect prior to reconstruction. According to Millard, "If possible make the defect fit the flap or graft to that unit!"
Principle III: Always Have a Pattern and a Back-up Plan
As with all surgery, comparing the pros and cons of each surgical option is of the utmost importance. The reconstructive ladder is a mental exercise that provides the surgeon with options ranging from the simplest to most complex. Usually, things should be kept as simple as possible. This benefits both the surgeon and the patient; the simplest plan is often the safest.
However, physicians should not sell themselves or patients short. Avoid settling for the simplest procedure just for the sake of simplicity. More complex problems may require more complex solutions, and the simplest approach may be, frankly, inadequate. A sound plan must provide restoration of function and aesthetic form; these are the fundamental goals of plastic and reconstructive surgery.
A nose, breast, [9] or finger reconstruction should be designed to fit its use and location, rather like the philosophy used by architects when designing buildings. In 1949, a pioneer of 20th-century architecture, Frank Lloyd Wright, said, "Form and function thus become one in design and execution if the nature of materials and methods and purposes are all in unison." Several years earlier, Wright had been asked to build a hotel in Tokyo. As Japan was in an earthquake zone, Wright designed the hotel to withstand shocks using a sea of mud to support the foundations. Following the Japanese earthquake of 1923, Wright's hotel was apparently the only building left standing in Tokyo.
Once a plan has been determined, rehearse it. Trace the defect or cut a pattern to fit the defect. Transpose the pattern and experiment with it to decide on the best donor area and orientation. Omitting this step is akin to Wright building his hotel without a blueprint, and his materials were much cheaper than the surgeon's.
Finally, the surgeon should ask him or herself "what do I do next if this fails?" Proceed to the operating room only after answering this question definitively. Once in the operating room, keep an open mind and be ready to adjust the surgical plan as the situation dictates.
Principle IV: Steal From Peter to Pay Paul
Apply the principle "steal from Peter to pay Paul, but only when Peter can afford it." Using what the body has to reconstruct a deficit is essentially "robbing the bank." The goal to achieve is ultimate efficiency, or, according to Millard, "getting something for almost nothing." Examples of local flaps illustrating Millard's point are shown in the images below.
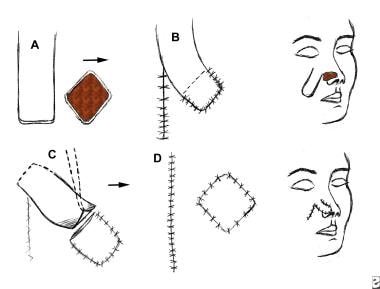 An interpolated flap. The donor site is separated from the recipient site, and the pedicle of the flap must pass above or beneath the tissue to reach the recipient area. (A) Flap is outlined and elevated. (B) Donor site is closed. (C) Pedicle is divided once the flap is revascularized. (D) Insetting of the flap is completed.
An interpolated flap. The donor site is separated from the recipient site, and the pedicle of the flap must pass above or beneath the tissue to reach the recipient area. (A) Flap is outlined and elevated. (B) Donor site is closed. (C) Pedicle is divided once the flap is revascularized. (D) Insetting of the flap is completed.
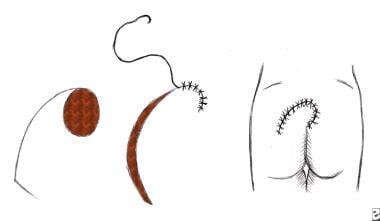 A rotation flap. Movement is in the direction of an arc around a fixed point and primarily in one plane. This is a semicircular flap.
A rotation flap. Movement is in the direction of an arc around a fixed point and primarily in one plane. This is a semicircular flap.
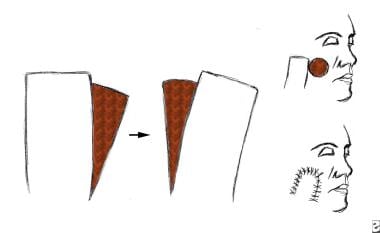 A transposition flap. The rectangular flap is rotated on a pivot point. The more the flap is rotated, the shorter the flap becomes.
A transposition flap. The rectangular flap is rotated on a pivot point. The more the flap is rotated, the shorter the flap becomes.
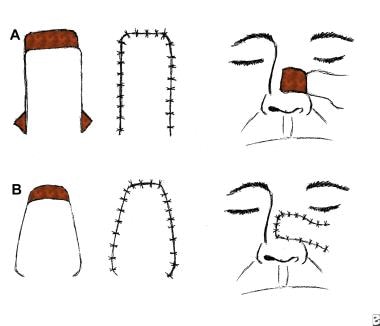 An advancement flap. Advancement flaps are moved primarily in a straight line from the donor site to the recipient site. No rotational or lateral movement is applied. Triangles y (Burrow triangles) have been removed lateral to the base equal to the distance of the advancement (x = y). Incisions are made into the base of the flap to assist in the advancement.
An advancement flap. Advancement flaps are moved primarily in a straight line from the donor site to the recipient site. No rotational or lateral movement is applied. Triangles y (Burrow triangles) have been removed lateral to the base equal to the distance of the advancement (x = y). Incisions are made into the base of the flap to assist in the advancement.
Do not make the naive mistake of merely advancing tissue to the deficient area unless this can be accomplished completely without tension. Tension compromises the blood supply of the advanced tissue and, ultimately, results in flap failure. Tension is to be feared the most. It must be recognized and prevented or else used to the surgeon’s advantage.
A study by Sadigh et al described the effective use of a recycle flap, in which soft tissue is harvested from an existing flap, allowing debulking and refinement of the original flap while creating a second flap that requires no additional donor site. Of 60 recycle flaps (including 29 random flaps, 29 pedicled perforator flaps, and two free perforator flaps), 58 survived completely. Necrosis occurred in two perforator flaps created from previously irradiated flaps. [10]
Principle V: Never Forget the Donor Area
Surgeons once believed in treating the primary defect without worrying about the secondary defect. Plastic surgery has since progressed. Plastic and reconstructive surgeons now realize the importance of considering both defects equally. [11]
The reality is that it is not possible to get something for nothing; a price usually must be paid following reconstruction of a primary defect. The significance of providing coverage of a defect with minimal deformity and disability is one of the foremost principles on which the reconstructive surgery specialty is based.
If reconstruction of the primary defect is too costly in terms of resultant deformity or disability, reevaluate and use another reconstructive option.
Remember that donor areas are not limitless. One cannot continuously use tissue without paying back in some way. Carelessness or overuse of a donor area eventually causes damage that may be far greater than the original defect.
A study by Kansy et al assessing morbidity at frequent microvascular donor sites for head and neck reconstruction—the radial forearm, anterolateral thigh, fibula, iliac crest, and scapula—found that out of 117 cases, 60% experienced reduced sensation at the donor site; 49.6%, reduced muscle strength; and 12.8%, restricted range of motion, with 31.2% of patients experiencing limitations in daily activities. The incidence of overall donor site morbidity was greatest in association with scapula flaps. [12]
A survey study by McLeod and Islur indicated that in free flap reconstruction, patients would prefer, in terms of aesthetics, that the flap be harvested from the leg or back. Participants demonstrated that for small reconstructions, donor site scars on the leg or back were preferable to those on the arm or abdomen. For medium-sized reconstructions, the survey revealed a preference for back and upper thigh scars, as opposed to abdominal and anterior thigh scars. Midline abdominal scars were considered the least preferable with regard to small and medium-sized reconstructions. Back, thigh, and lower abdominal donor sites were preferred for large reconstructions. Survey preferences may have been associated with the ability to hide scars beneath clothing. [13]
Final Thoughts
See the list below:
Be thoughtful. Consider all options, simple to complex, prior to any flap surgery.
Be knowledgeable. Know and understand the anatomy, blood supply, and quality of tissue available.
Be prepared for failure. Have a back-up plan available in case the first plan fails.
Guidelines
The United Kingdom National Multidisciplinary Guidelines regarding head and neck cancer, published in 2016, include the following recommendations for reconstruction following ablative surgery [14] :
-
Microsurgical free flap reconstruction should be the primary reconstructive option for most defects of the head and neck that need tissue transfer
-
Free flaps should be offered as first choice of reconstruction for all patients needing circumferential pharyngoesophageal reconstruction
-
Free flap reconstruction should be offered for patients with class III or higher defects of the maxilla
-
Composite free tissue transfer should be offered as first choice to all patients needing mandibular reconstruction
-
Patients undergoing salvage total laryngectomy should be offered vascularized flap reconstruction to reduce pharyngocutaneous fistula rates
-
Patterns of muscle flap vascular anatomy. Type I - One vascular pedicle. Type II - Dominant pedicle(s) and minor pedicle(s). Type III - Two dominant pedicles. Type IV - Segmental vascular pedicles. Type V - One dominant pedicle and secondary segmental pedicles.
-
An interpolated flap. The donor site is separated from the recipient site, and the pedicle of the flap must pass above or beneath the tissue to reach the recipient area. (A) Flap is outlined and elevated. (B) Donor site is closed. (C) Pedicle is divided once the flap is revascularized. (D) Insetting of the flap is completed.
-
A rotation flap. Movement is in the direction of an arc around a fixed point and primarily in one plane. This is a semicircular flap.
-
A transposition flap. The rectangular flap is rotated on a pivot point. The more the flap is rotated, the shorter the flap becomes.
-
An advancement flap. Advancement flaps are moved primarily in a straight line from the donor site to the recipient site. No rotational or lateral movement is applied. Triangles y (Burrow triangles) have been removed lateral to the base equal to the distance of the advancement (x = y). Incisions are made into the base of the flap to assist in the advancement.
-
A bipedicle flap with incisions parallel to the advancement.
-
A V-Y advancement flap.
Tables
What would you like to print?
- Classification and Principles of Flap Surgery
- Flap History and Classification
- Principle I: Replace Like With Like
- Principle II: Think of Reconstruction in Terms of Units
- Principle III: Always Have a Pattern and a Back-up Plan
- Principle IV: Steal From Peter to Pay Paul
- Principle V: Never Forget the Donor Area
- Final Thoughts
- Guidelines
- Show All
- Media Gallery
- References