Overview
Embryology, the study of animal development, has been the subject of scientific curiosity for thousands of years. Some of the earliest recorded investigations were performed on the chick embryo by the Greek philosopher Aristotle. The fusion of 2 gametes, the sperm and the egg, is termed fertilization. The fertilized egg, or zygote, subsequently divides, differentiates, and maturates into the neonate that is received at birth. This process is extremely complex but proceeds in a regular, stepwise fashion to its completion.
The mammary glands are a distinguishing feature of mammals and a primary symbol of femininity in our culture. Breastfeeding provides nourishment and passive immunity to offspring, promotes postpartum uterine involution, and establishes an important bond between mother and infant. The mammary gland begins development early in embryologic life and only culminates in the postpartum lactation of the adult female. An understanding of the development of the mammary gland is essential to anyone asked to evaluate and treat aberrancies of such development.
Congenital breast malformations can be extremely psychologically debilitating to the individual. Accurate diagnosis, counseling, and treatment are necessary to alleviate the sense of deformity and unattractiveness that is often present. Proper timing of surgical intervention is necessary to optimize functional, psychological, and aesthetic outcomes. This article describes normal embryologic mammary gland development and the more common congenital malformations that may confront the plastic surgeon.
The Integument
The integument consists of 2 layers, the epidermis and dermis, which form a variety of structures that allow us to interact with our environment. The epidermis is derived primarily from surface ectoderm but is colonized by pigment-containing melanocytes of neural crest origin, antigen-processing Langerhans cells of bone marrow origin, and pressure-sensing Merkel cells of unclear origin. The dermis is derived primarily from mesoderm and contains many blood vessels and sensory structures.
During the fourth week of embryologic development, the single cell thick ectoderm and underlying mesoderm begin to proliferate and differentiate into the definitive multi-layered skin present at birth. The specialized structures formed by the skin, including teeth, hair, hair follicles, fingernails, toenails, sebaceous glands, eccrine glands, apocrine glands, and mammary glands, also begin to appear at approximately this period in development. Teeth, hair, and hair follicles are formed by the epidermis and dermis in concert, while fingernails and toenails are formed by the epidermis alone.
Sebaceous glands, eccrine glands, apocrine glands, and mammary glands are considered epidermal glands in that they develop as downgrowths or diverticula of the epidermis into the dermis. Sebaceous glands, or holocrine glands, are found over the entire surface of the body except the palms, soles, and dorsum of the feet. They are largest and most concentrated in the face and scalp where they are the site of origin of acne. The normal function of sebaceous glands is to produce sebum, a group of complex oils including triglycerides and fatty acid breakdown products, wax esters, squalene, cholesterol esters, and cholesterol.
Eccrine sweat glands are found over the entire surface of the body except the lips, external ear canal, and labia minora. They are most concentrated in the palms and soles of the feet. The normal function of eccrine glands is to produce the water and electrolyte component of sweat, which cools the body by evaporation.
Apocrine sweat glands are similar in structure but not identical to eccrine glands. They are concentrated in the axillae and anogenital regions. They probably serve a vestigial sexual function, because they produce odor and do not function prior to puberty. The mammary gland is considered to be a modified and highly specialized type of apocrine gland.
The Embryologic Breast
Embryologic development of the mammary gland consists of a series of highly ordered events involving interactions among a number of distinct cell types. These interactions are regulated by an array of systemic and local factors such as growth factors and hormones. [1, 2] Development is initially identical among males and females of the same species.
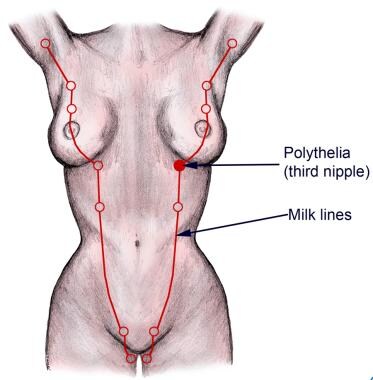
During the fourth week of gestation, paired ectodermal thickenings termed mammary ridges or milk lines develop on the ventral surface of the embryo and extend in a curvilinear fashion convex towards the midline from the axillae to the medial thigh. This is the first morphologic evidence of mammary gland development. In normal human development, these ridges disappear except at the level of the fourth intercostal space on the anterior thorax, where the mammary gland subsequently develops. In other species, such as cats and dogs, multiple paired mammary glands develop along the mammary ridges in the chest, abdominal, and groin regions. The number of paired glands varies greatly among mammalian species and is related to the number of offspring in each litter.
During the fifth week of gestation, the remnant of the mammary ridge ectoderm begins to proliferate and is termed the primary mammary bud. This primary bud subsequently begins growth downward as a solid diverticulum into the underlying dermis during the seventh week. By the 10th week, the primary bud begins to branch, yielding secondary buds by the 12th week, which eventually develop into the mammary lobules of the adult breast.
This initial downgrowth and subsequent branching has been shown to occur as the result of an inductive influence of the extracellular matrix of the mesoderm on the primary mammary bud. This epithelial-mesenchymal signaling is probably through paracrine and juxtacrine mechanisms where the underlying mesoderm produces growth factors and hormones that interact with receptors on the overlying ectodermal cells of the primary mammary bud. The adipose tissue in the underlying mesoderm represents a significant store of lipids for the production of hormones and growth factors, which are then available to promote and regulate growth of the developing mammary gland.
During the remainder of gestation, these buds continue lengthening and branching. During the 20th week, small lumina develop within the buds that coalesce and elongate to form the lactiferous ducts. The canalization of the mammary buds with formation of the lactiferous ducts is induced by placental hormones entering the fetal circulation. These hormones include progesterone, growth hormone, insulinlike growth factor, estrogen, prolactin, adrenal corticoids, and triiodothyronine. At term, approximately 15-20 lobes of glandular tissue have formed, each containing a lactiferous duct. Support for the breast comes from both the skin envelope and the fibrous suspensory ligaments of Astley Cooper that anchor the breast to the pectoralis major fascia.
The lactiferous ducts drain into retroareolar ampullae that converge into a depressed pit in the overlying skin. Each of the 15-20 lobes of the mammary gland has an ampulla with an orifice opening into this mammary pit. Stimulated by the inward growth of the ectoderm, the mesoderm surrounding this area proliferates, creating the nipple with circular and longitudinally oriented smooth muscle fibers. The surrounding areola is formed by the ectoderm during the fifth month of gestation. The areola also contains other epidermal glands, including glands of Montgomery (sebaceous glands that serve to lubricate the areola).
The Neonatal Breast
At birth, the breast is composed of radially arranged mammary lobes draining via lactiferous ducts into ampullae that empty onto the nipple. These rudimentary mammary glands are identical in males and females. The nipple appears as a small pit in the center of a thickened areola containing a few glands of Montgomery. Shortly after birth, the nipples become everted from proliferation of the surrounding mesoderm, and the areolae develop a slight increase in pigmentation. The development of erectile tissue in the nipple areolar complex causes the nipple to protrude even further upon stimulation.
Failure of the nipples to evert may indicate the presence of inverted nipples, which is usually caused by fibrous bands and a hypoplastic ductal system tethering the nipple in the inverted position. This condition is familial in 50% of patients, may occur in males and females, and is only clinically significant in that it may present a mechanical problem for women attempting to breastfeed an infant. The new onset of inverted nipples in adolescence or adulthood may indicate infection or malignancy.
Falling levels of maternal estrogens in the neonatal bloodstream stimulate the neonatal pituitary hypophysis to produce prolactin. This results in unilateral or bilateral breast enlargement in as many as 70% of neonates. Histologically, this appears as hypertrophy of the ductal system. Acini appear and an increase in vascularity of the gland is observed. This is often accompanied by the secretion of witch's milk, a cloudy fluid similar to colostrum consisting mainly of water, fat, and cellular debris. These changes occur equally among male and female neonates and regress spontaneously within several weeks as neonatal prolactin production declines and the glands become quiescent. However, attempting to strip the breasts of their witch's milk, as advocated by some superstitions, can lead to a persistent hyperplastic secretory state lasting many months.
The Male Breast
Embryologic breast development and the transient secretory phase of the neonatal breast occur in male and female breasts. However, important developmental differences should be noted. Although the early stages of development are independent of sex steroid hormones, the mammary glands become extremely responsive to their hormonal environment. The presence of testosterone and its binding to mesodermal receptors leads to the normal involution of the male mammary gland. However, in testicular feminization syndrome, where circulating testosterone but an absence of testosterone receptors occurs, the individual develops a female phenotype, including typical female breast development.
Approximately two thirds of males develop some degree of hyperplasia of the breasts, or gynecomastia, during puberty. This condition persists for several months to several years and will spontaneously resolve in all but approximately 7% of those affected. The etiology is usually a decreased ratio of testosterone to estradiol, and when testicular function becomes dominant, the hypertrophy resolves. Although temporary in most instances, this condition can be psychologically devastating to a teenage boy who may be teased by his peers and, as a result, becomes unwilling to change in gym class or remove his shirt at the pool or beach.
During this period, the patient or his family may seek consultation and treatment. Reassurance that the condition is most likely temporary provides psychological support and hopefully avoids premature surgical intervention. Use of certain drugs, namely H2 blockers, anabolic steroids, marijuana, and heroin, can prolong the presence of gynecomastia in this population. Gynecomastia also is seen in approximately 40% of males with Klinefelter syndrome (trisomy of the sex chromosomes [XXY]). [3]
Treatment of gynecomastia without underlying cause that does not resolve spontaneously within several years is relatively simple. Because of the elasticity of the skin in this population, a combination of liposuction with direct subareolar excision techniques is generally successful. Older patients with less elastic skin may require more standard breast reduction techniques. Obese patients may benefit from preoperative weight loss, but still require excision of some sort. For more details on gynecomastia, please see the Medscape Reference article Gynecomastia.
Supernumerary breast tissue and the absence of breast tissue also occur in males and are discussed with other congenital breast malformations in this article.
The Female Breast
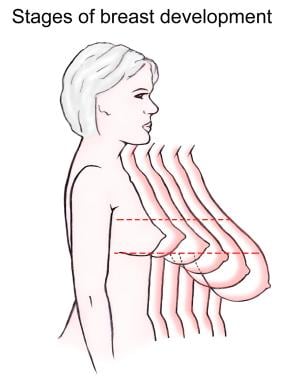
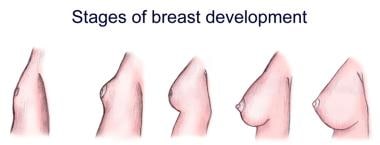
The breast undergoes 3 major cycles of development. The initial phase occurs between birth and puberty, during which time the breast stroma and glandular tissue increase gradually.
Development of the mammary glands is initiated during embryologic life but is only complete in the postpartum lactation of the adult female. [4] After the transient secretion stimulated by prolactin production in the neonate, the mammary glands, with their relatively simple architecture, remain quiescent until puberty. During this period, the supporting stromal structures and ducts enlarge in proportion to the increase in body size of the individual, but no lobular development occurs.
Thelarche, the rapid growth that occurs at the onset of puberty, is primarily from deposition of fat and development of periductal connective tissue, but elongation and thickening of the ductal system also occurs at this stage. Ductal growth occurs under the influence of circulating estrogens, growth hormone, and prolactin but is independent of progesterone. The development initiated at the onset of puberty is generally complete by age 20 years.
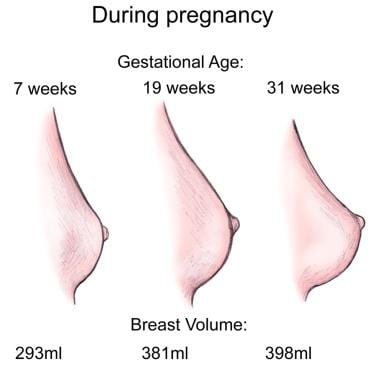
If pregnancy occurs, the glands complete their differentiation and reach functional maturity with the intralobular-branched ducts forming buds that become secretory alveoli. This occurs under the influence of sustained increases in the levels of circulating progesterone, estrogens, prolactin, and placental lactogen. The epithelial cells of the alveoli begin to accumulate the cytoplasmic organelles necessary to sustain lactation in the postpartum period.
Following parturition, lactation proceeds as a response to environmental stimuli and the withdrawal of circulating progesterone. Prolactin, originating in the anterior pituitary, and somatomammotropin, originating in the placenta, stimulate the alveolar epithelium to produce and secrete milk proteins, primarily casein and alpha-lactalbumin, and lipids. In response to suckling or crying, oxytocin produced by the hypothalamus but released by the posterior pituitary causes contraction of myoepithelial cells surrounding the alveoli, which expels the milk to assist the suckling child.
With cessation of nursing, milk production also ceases because of a decrease in circulating prolactin and the inhibitory effects of nonexpelled milk. The alveoli then return to their prior nonfunctioning state. By age 40 years, the mammary glands begin to atrophy. During and after menopause, the altered hormonal environment leads to a senescent state, with involution of the glandular component and replacement with connective tissue and fat.
Congenital Breast Malformations
Congenital breast malformations range in severity from the relatively minor to major chest wall deformities. Minor malformations may not even be recognized, while major deformities may cause significant functional, psychological, and aesthetic concerns. The affected individual may present for consultation at any age, often early in childhood as a result of parental concern. It is important to be able to counsel the patient and his or her family regarding the nature of the problem, its prognosis for future development, and the appropriate indications and timing of surgical intervention. These malformations generally fall into 1 of 2 categories, the presence of supernumerary breast tissue or the absence or underdevelopment of breast tissue.
Supernumerary breast tissue
The presence of supernumerary breast tissue indicates incomplete involution of the milk line, resulting in the formation of accessory mammary tissue from the redundant clusters of ectopic primordial breast cells. [5] This occurs in 2-6% of females and 1-3% of males. Approximately one third of affected individuals have more than one site of supernumerary breast tissue development. Most of this accessory breast tissue has no physiologic significance, but some may enlarge with the onset of puberty, pregnancy, or lactation, and can be the site of breast carcinoma.
Approximately 67% of accessory breast tissue occurs in the thoracic or abdominal portions of the milk line, often just below the inframammary crease and more often on the left side of the body. Another 20% occurs in the axilla. The remaining locations include anywhere along the milk line or in the buttock, back, face, and neck. Supernumerary tissue present in any location other than along the milk line represents a migratory arrest of breast primordium during chest wall development.
In 1915, Kajava published a classification system for supernumerary breast tissue that remains in use today. Class I consists of a complete breast with nipple, areola, and glandular tissue. Class II consists of nipple and glandular tissue but no areola. Class III consists of areola and glandular tissue but no nipple. Class IV consists of glandular tissue only. Class V consists of nipple and areola but no glandular tissue (pseudomamma). Class VI consists of a nipple only (polythelia). Class VII consists of an areola only (polythelia areolaris). Class VIII consists of a patch of hair only (polythelia pilosa).
In a study of 11 girls aged 13-16 years with axillary ectopic breast tissue (Kajava class IV or V), De la Torre et al found that this tissue manifested as a 2-5 cm mass, with “associating cyclic pain with menstruation (45%) and fluctuating volume (36%).” The ectopic tissue was bilateral in two of the girls (18.2%). The patients assessed the clinical and aesthetic results of open excision of the tissue as “very good.” [6]
The most common form of supernumerary breast tissue is polythelia, the presence of more than 2 nipples on an individual. [7, 8] More than 90% of supernumerary nipples occur in the inframammary region. This condition is present in 2-5% of the general population, although many additional patients may go undiagnosed because the nipple is often confused for a nevus or other benign skin lesion because of its diminutive size. Males and females have an overall equal incidence, but differences are observed within ethnic groups. For example, polythelia is present in 5% of Japanese females but only 1.6% of Japanese males. Differences also exist among ethnic groups. Polythelia occurs less frequently in Caucasians (0.6%) than in blacks (3.5%). Most cases are sporadic, but approximately 6% are familial and are believed to represent an autosomal dominant trait with variable penetrance.
A correlation exists between renal disease and polythelia. Nephrologic abnormalities such as cysts, duplications, or unilateral renal agenesis have been found in 14.5% of sporadic cases and 32.1% of familial cases compared to 1-2% of the general population. Approximately 19% of patients with renal adenocarcinoma have polythelia, and 16.5% of patients with end-stage renal disease have polythelia. Considering the significant incidence of congenital and acquired renal disorders in patients with polythelia, patients should be aware of the need for regular physical examination and urinalysis. Any abnormality noted should alert the physician to the need for a renal ultrasound.
Polymastia, the presence of accessory glandular tissue, is the second most common form of supernumerary breast tissue, occurring in 1-2% of the female population. Various forms exist, as described by Kajava Classes I through IV, but, most commonly, the nipple and areola are absent or rudimentary. The most common location is in the axilla, where they may present as axillary fullness responsive to hormonal cycles of menstruation, pregnancy, or lactation. The second most common location is in the inframammary region, similar to polythelia. Most cases are sporadic, but this condition also has been observed as a heritable trait.
The presence of supernumerary tissue can be psychologically disturbing to adolescents. Excision is recommended prior to puberty or at any age when the condition is recognized and becomes of concern to the individual.
Absence or underdevelopment of breast tissue
The absence or underdevelopment of breast tissue is less common than the presence of supernumerary tissue. These conditions may be unilateral or bilateral and result from partial or complete underdevelopment of the mammary bud. The most severe form is amastia, the complete absence of glandular tissue, nipple, and areola. Hypoplasia, the presence of very small rudimentary breasts, is the most common form. Amastia and hypoplasia may be associated with scalp defects, ear abnormalities, renal hypoplasia, and cataracts in patients with the rare autosomal dominant Finlay-Marks syndrome. [9] Hypoplasia also may occur in patients with Turner syndrome (ovarian agenesis), congenital adrenal hyperplasia, and delayed menarche, where the administration of oral estrogen therapy usually promotes glandular development.
Aplasia, the absence of glandular tissue in the presence of a nipple and areola, is most commonly encountered in Poland syndrome, first described in 1841. This condition is often accompanied by musculoskeletal deformities of the chest wall and ipsilateral upper extremity and is discussed in a separate article. Athelia, absence of the nipple and areola in the presence of glandular tissue, is the rarest of these conditions. It is infrequently seen as an isolated defect except in ectodermal dysplasia syndromes.
The goal of surgical therapy in these patients is to achieve breast symmetry. This may best be performed in late adolescence when the contralateral breast has reached its mature size and shape. However, earlier intervention may be indicated in the patient with a significant sense of deformity that is adversely affecting body image.
Techniques to achieve symmetry may include prosthetic devices such as implants or expanders, autologous tissue such as the latissimus dorsi or rectus abdominis muscles, or both. [10] These patients and their families should seek plastic surgery consultation early in the process so all options can be explained to them. A study by Derder et al suggested that lipofilling may be an effective treatment for congenital hypoplastic breast anomalies. The study, which involved 10 patients below age 20 years (with Poland syndrome, bilateral tuberous breast, or unilateral micromastia), found that almost all patients had satisfactory cosmetic results at 1-year follow-up and that patients’ postprocedure cup sizes remained the same after a follow-up period of 5 years or more. [11, 12]
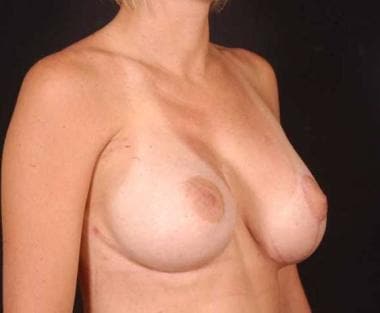 Postoperative side view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
Postoperative side view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
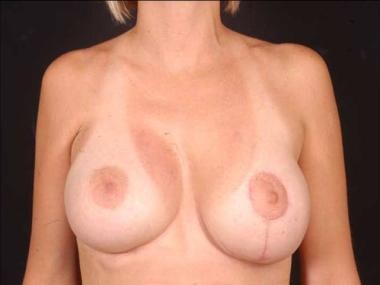 Postoperative frontal view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
Postoperative frontal view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
Conclusion
The mammary gland is a complex organ that begins development early in gestation. The extensive research currently being performed on the human genome, molecular biology, and growth factors will certainly contribute to the further elucidation of the embryologic factors involved in the formation, differentiation, and development of the mammary gland. This may provide important insights into the cause, treatment, and potential prevention of mammary gland abnormalities such as congenital malformations and breast cancer. Given that breast cancer strikes 10% of women in the United States and congenital malformations occur in 2-6%, these developments may have enormous implications for the future of medicine.
-
Natural milk lines.
-
Stages of breast development.
-
Breast changes during pregnancy.
-
Stages of breast development.
-
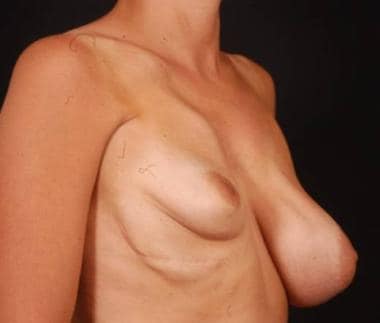
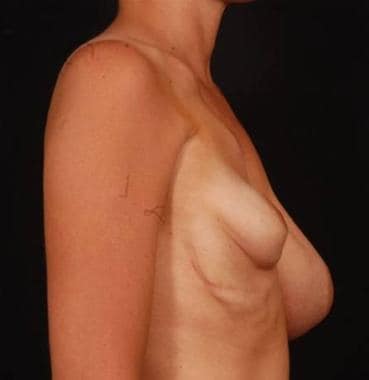
Preoperative frontal view of patient with tuberous breast deformity.
-
Preoperative side view of patient with tuberous breast deformity.
-
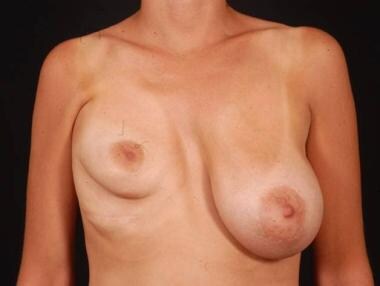
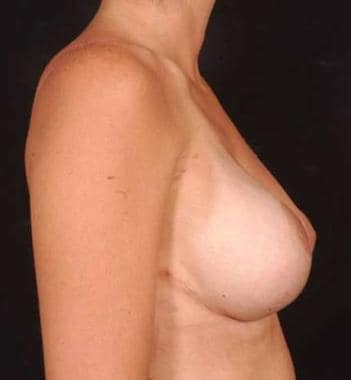
Postoperative frontal view of the patient with tuberous breast deformity shown in media files 5-6. This patient underwent bilateral reconstruction with silicone gel implants.
-
Postoperative side view of the patient with tuberous breast deformity shown in media files 5-6. This patient underwent bilateral reconstruction with silicone gel implants.
-
Preoperative side view of patient with Poland syndrome.
-
Preoperative frontal view of patient with Poland syndrome.
-
Preoperative profile view of patient with Poland syndrome.
-
Postoperative side view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
-
Postoperative frontal view of patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.
-
Postoperative profile view of the patient with Poland syndrome shown in media files 9-11. This patient underwent reconstruction with a form stable gel device in the right breast and a nonform stable gel device in the left breast.