Overview
Background
A competent and dynamically functional velopharyngeal sphincter is essential for normal eating, normal breathing, and intelligible speech. The sphincter is positioned between the oral and nasal cavities and coordinates appropriate airflow through each chamber to allow a voice that has quality, richness, and carrying power. Closure of the velopharyngeal port prevents nasal regurgitation during eating and allows pronunciation of consonants, while opening of the port allows for normal respirations and specific nasal articulations.
The terms velopharyngeal dysfunction (VPD), velopharyngeal incompetence, velopharyngeal insufficiency (VPI), and velopharyngeal inadequacy are frequently used to denote an improperly functioning velopharynx. VPD includes any structural and/or neuromuscular disorder of the velum and/or pharyngeal walls at the level of the nasopharynx that results in interference of the normal sphincteric closure. It may result from anatomic, myoneural, behavioral, or a combination of disorders. (See Velopharyngeal Dysfunction.)
Because this article focuses primarily on pharyngoplasty and pharyngeal flaps, the term “velopharyngeal dysfunction” is used to refer specifically to speech and resonance symptoms related to a known structural deficit that has been determined by perceptual and instrumental differential diagnosis. VPD is diagnosed clinically by a constellation of symptoms that includes pathologically incurred nasal resonance (hypernasality), compensatory misarticulations, escape of air through the nose (nasal emissions), and aberrant facial movements (grimacing).
Hypernasality is a quality perceived by the listener due to inappropriate nasal coupling, which refers to the balance of air traveling through the oral and nasal airways during speech. Errors in articulation of vowels and consonants occur from an inability to produce appropriate high intraoral pressure consonants, specifically, the stops, fricatives, and affricates. Tests such as the modified Müller maneuver cannot help distinguish patients with hypernasality.
Stop plosives (ie, p, b, t, d, k, and g as in “go”) are brief, purposeful interruptions of the airstream resulting in intraoral pressure buildup, with explosive release of that pressure buildup. Fricatives refer to sounds (s, z, sh, zh, f, v, and both voiced and voiceless th) produced by constricting the airstream through a narrow channel between the 2 speech articulators. Affricates (ch and g as in “George”) are high intraoral pressure consonants that begin as stops, but are released as fricatives.
Several interventions are available for management of VPD, depending on the differential diagnosis and the cause of the pathology.
Go to Velopharyngeal Dysfunction for complete information on this topic.
Indications
Patients with symptoms of VPD (eg, hypernasality, nasal emission, facial grimacing, compensatory misarticulations) are referred to a multidisciplinary velopharyngeal diagnostic center for video-recorded standard perceptual, nasoendoscopic, and fluoroscopic speech evaluations. [1] The videos provide direct visualization of velopharyngeal closure for review by the interdisciplinary VPD team, and a consensus is reached for recommended management.
Candidates for surgery fulfill the criterion of VPD dysfunction resulting from an anatomic, myoneural, or combined deficiency of the velopharyngeal sphincter that is not expected to be managed by speech therapy alone.
Contraindications
To stimulate collapse of the pharyngeal walls in patients with obstructive sleep apnea, have the patient perform a modified Müller maneuver. Instruct the patient to breathe rapidly with the mouth closed and the nose partially closed. Surgery to decrease the velopharyngeal port is contraindicated in these patients.
Velopharyngeal-narrowing procedures are not appropriate for patients who meet the following criteria:
-
The patient declines surgical management by choice
-
The patient has a known or suspected risk for potential airway obstruction (patients with known obstructive sleep apnea or other upper airway compromise must be approached with caution with regard to performing posterior pharynx surgery; because of the possibility of triggering apneic episodes, pharyngeal flaps can be dangerous if performed in these patients; patients requiring surgical management who have risk factors for upper airway obstruction are preferentially recommended for sphincter pharyngoplasty on the basis of reports of its minimal effect on the airway)
-
The patient has intermittent or inconsistent closure that responds well to speech therapy
-
The patient has incomplete diagnostic results; with further studies and improvements in diagnostic technologies, speech production disorders should be more accurately assessed and individually managed to achieve optimal results
In addition, syndromic patients (ie, those with velocardiofacial syndrome [VCF syndrome]) may have anomalous carotid artery anatomy, which places these structures within the operative field of posterior pharynx surgery. However, in the view of some authors, visible pulsations on the posterior pharyngeal wall, indicating aberrant carotid arteries (see Anatomic Considerations), should not be considered an absolute contraindication to surgery. [2, 3, 4]
A plethora of organizations provide humanitarian assistance to developing countries in which cleft care is not organized or coordinated. Most medical missions are undertaken on a short-term basis, which means that intermediate- and long-term outcome assessments are not performed. Because velopharyngeal-narrowing procedures have significant potential airway morbidity, arguably they should not be performed in the setting of a foreign medical mission.
Pathophysiology and etiology
Causes of VPD can be divided into 2 main categories as follows:
-
Structural deficiency of the palate or its related musculature (eg, cleft palate)
-
Neurologic deficiency (eg, myasthenia gravis, cerebrovascular accident, upper or lower motor neuron lesions, head trauma)
Peterson-Falzone discusses noncleft causes of VPD, such as neuromuscular disorders, palatopharyngeal disproportion, and the effects of tonsils and adenoids. [5] The degree to which each component contributes to VPD is difficult to determine.
Anatomically, VPD may also be divided into 2 categories as follows:
-
Poor mobility of the soft palate secondary to various factors, including short palate, scarred/tightened palate, or dysfunction/malposition of the levator palatini muscle
-
Poor mobility of the posterior and lateral pharyngeal walls due to poor muscular mobility
The latter is more commonly caused by a neurologic deficit.
Anatomic considerations
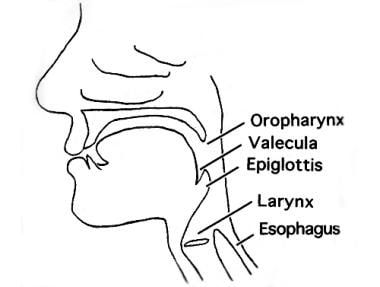
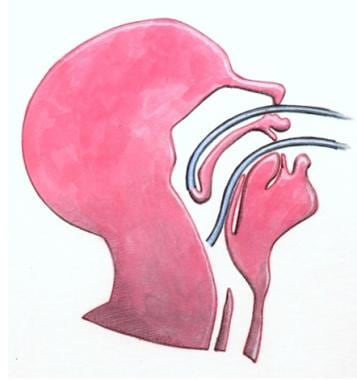
Surgical treatment of VPD rests on an understanding of the anatomy of the velopharynx (ie, the palate, posterior pharyngeal wall, and airway; see the image below).
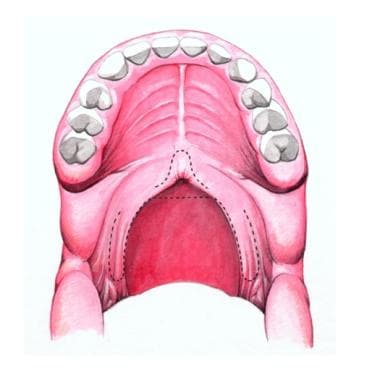
The soft palate marks the beginning of the oropharynx and is the movable posterior third of the palate. It forms an incomplete septum between the mouth and the pharynx. It is marked by a median raphe and is continuous with the roof of the mouth and the mucous membrane of the nasal floor (see the image below).
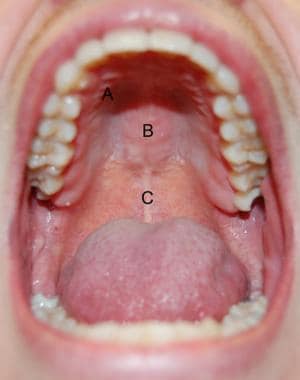 Hard and soft palates. A: transverse rugae of hard palate; B: median raphe of hard palate; C: median raphe of soft palate.
Hard and soft palates. A: transverse rugae of hard palate; B: median raphe of hard palate; C: median raphe of soft palate.
The composite movements of the lateral pharyngeal walls, the velum, and the posterior pharyngeal walls close the velopharyngeal port in deglutition and during oral speech sounds; they open the port for breathing and some nasalized articulations. Patterns of closure as observed on preoperative instrumental assessments include coronal, sagittal, bow-tie, circular, and Passavant (see the image below).
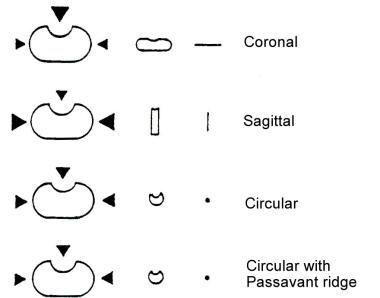 Schematic bird's-eye view of velopharynx, illustrating directional movements of representative closure patterns.
Schematic bird's-eye view of velopharynx, illustrating directional movements of representative closure patterns.
The 3 muscles of the palate (levator veli palatini, tensor veli palatini, and uvularis) work in concert with the palatopharyngeus, the palatoglossus, and the pharyngeal constrictor muscles to produce VP closure.
The tensor veli palatini muscles arise from the membranous wall of the eustachian tube. Their tendons pass around the hamular processes of the medial pterygoid plate of the sphenoid and insert into the palatine aponeurosis. The levator veli palatini muscles also have their origin along the eustachian tube orifice. They meet in the midline in a sling-like fashion above and behind the aponeurosis. The uvularis is a small midline muscle sitting above and behind the levator sling.
An unimpaired velum moves posteriorly and superiorly, the posterior pharyngeal wall can move ventrally diffusely or as a well-defined shelf (known as the Passavant ridge), and the lateral pharyngeal walls move toward the midsagittal midline. The uvularis also contracts during speech, adding bulk to the area of convexity on the upper surface of the soft palate. The adenoids, residing in the posterior pharyngeal wall, and the pharyngeal tonsils, on the lateral pharyngeal walls, may augment or interfere with the function of those walls in velopharyngeal closure. [6]
As a person prepares to speak, the velum is partially raised and held at the ready position before speech begins; it then moves to the closed position as phonation starts. For nasal sounds (eg, “m, n”), the sphincter remains open. The ability of the sphincter to close is essential for compression of air behind the point of constriction so that consonants, especially plosives (eg, “f, s, th”) can release with sufficient strength.
For more information about the relevant anatomy, see Mouth Anatomy and Pharynx Anatomy.
Aberrant carotid arteries
Anomalous internal carotid arteries have been shown to be a frequent feature of VCF syndrome. These vessels pose a potential risk for iatrogenic injury and hemorrhage during velopharyngeal-narrowing procedures. Various forms of cervical vascular imaging studies (eg, computed tomography [CT] scanning and angiography) have been advocated for defining the preoperative vascular anatomy so as to assist surgery. Whether these studies alter the conduct or outcome of operations on the velopharynx remains unclear.
Iatrogenic injuries to the carotid artery during velopharyngeal surgery are strikingly absent in the extant literature. Occasionally, transmission of vascular pulsations through floppy redundant mucosa may artificially masquerade as an ominous vessel. Additionally, tortuous mesially displaced vessels observed at one point have been shown to straighten out laterally on later studies.
The issue of how surgeons should approach the problem of aberrantly located carotid vessels is provocative and controversial and deserves overt answers from each participating surgeon; however, safety must prevail as the first priority.
When displaced vessels are identified, surgeons are faced with 3 main options. First, the surgeon may simply abandon the procedure. Second, the surgeon may go ahead with the operation and “operate around” the displaced vessels. Third, the surgeon may choose to perform one procedure instead of another—for example, sphincter pharyngoplasty instead of pharyngeal flap. (Theoretically, performance of the latter procedure could expose a vessel over the full length of the flap.)
Some authors are more comfortable operating in the presence of these aberrant structures, provided that the flap(s) can be repositioned so as not to interfere with the structures, expose the vessel to oropharyngeal secretions, or compromise the execution of the procedure. Preoperative vascular imaging studies need not be routinely obtained on all patients. Awareness of the presence of aberrantly located carotid vessels comes from careful inspection of the small operative field, palpation of aberrant vessels intraoperatively, and cautious surgery.
Preparation
Preoperative evaluation
Preoperative consultations from appropriate subspecialists are recommended as needed by the velopharyngeal dysfunction (VPD) team (ie, speech and language pathologist, otolaryngologist, prosthodontist, and plastic surgeon).
Patients with symptoms of VPD (ie, hypernasality, nasal emission, facial grimacing, compensatory misarticulations) on perceptual speech screen are referred to a velopharyngeal diagnostic center for video-recorded standard perceptual, nasoendoscopic, and fluoroscopic speech evaluations.
The videofluoroscopic technique involves the instillation of barium into the nasopharynx. Real-time imaging can be used for playback at normal speed or slow motion. The images can be obtained in 3 dimensions (lateral, frontal, and basal). A major advantage of videofluoroscopy is its ability to help evaluate patients who are uncooperative or noncompliant. The examination usually takes 2-3 minutes, with radiation exposure of less than 0.02 Gy.
Videoendoscopy or nasopharyngoscopy uses a fiberoptic nasopharyngoscope with a high-intensity light attached to an endoscopic video monitor. [7] The procedure can be recorded on video. The scope is highly flexible and well tolerated (tip size of 3 mm). Topical anesthetic and phenylephrine are sprayed into the nasopharynx to establish mucosal decongestion and to induce superficial vessel vasoconstriction.
The nasopharyngoscope is placed through the nasal cavity superior to the velopharyngeal port, and the movements of the velum, the lateral pharyngeal walls, and the posterior pharyngeal wall are observed while the patient repeats oral speech targets that he or she can correctly articulate. In addition, the depth and width of the pharynx and abnormal morphology and distortions in movements of the palate and pharyngeal walls are evaluated.
The major advantage of videoendoscopy is the lack of ionizing radiation. This allows for a longer, more in-depth examination that can be repeated as often as necessary. Further, the ability of videoendoscopy to help assess all structures at the same time in relation to each other is superior to the 2-dimensional view obtained from videofluoroscopy.
The videos and patient records are reviewed by the interdisciplinary VPD team, and a consensus is reached for recommended management.
Initially on the basis of the history and physical examination, appropriate tests and procedures should be performed to confirm or rule out concomitant abnormalities of syndromic etiology.
Complication prevention
Pulsations of the posterior wall should alert the surgeon to possible anomalous internal carotid arteries that are placed too medial in location. Angiography should be performed to help with visualization and avoid injury to the vasculature.
To minimize the risk of surgically induced obstructive sleep apnea, a careful assessment of the upper airway, which may include formal sleep studies, is done before performing surgery.
Preoperative tonsillectomy or adenoidectomy
The tonsils and adenoids are often important components of the velopharyngeal closure mechanism. Occasionally, hypertrophic tonsils may herniate into the velopharyngeal port, so that lymphoid obstruction may actually be a source of speech dysfunction. At other times, enlarged tonsils may limit the technical placement of pharyngoplasty flaps or their sheer size may efface the myomucosal pillars, making flap elevation difficult.
Similarly, enlarged, friable, and hemorrhagic adenoids may inhibit performance of velopharyngeal surgery, and their presence may even compromise the outcome of surgical intervention if they contribute to flap dehiscence.
In these circumstances, preoperative tonsillectomy, adenoidectomy, or both may be indicated in advance of definitive surgical management of VPD. These procedures are performed 3 months before velopharyngeal surgery as needed to facilitate technical execution of the subsequent procedure.
The decision to perform tonsillectomy or adenoidectomy, however, must be made cautiously and in conjunction with the team otolaryngologist and speech pathologist. Tonsillectomy, and particularly adenoidectomy, should be avoided in any patient with symptoms of VPD until a differential diagnosis is established and a management plan is formulated by care providers and accepted by the patient and family.
Clinical manifestations of velopharyngeal insufficiency may worsen after tonsillectomy and adenoidectomy. Repeating the evaluation 3 months after these procedures is a wise plan because of potential changes in closure patterns that may alter the treatment plan as a result of such surgery.
If performing an adenoidectomy is necessary to facilitate the technical execution of velopharyngeal surgery, the patient and family must be duly warned about this predictable deterioration. It is advisable to wait for some time (eg, 3 mo) after adenoidectomy before proceeding with velopharyngeal surgery. Personally communicating with the team otolaryngologist to be certain he or she preserves the precious posterior tonsillar pillar tissue for later construction of the port is wise.
Technique
Overview
The goals of surgery are to eliminate the symptoms of hypernasality and eliminate audible nasal emissions without causing complete obstruction of the velopharyngeal port, allowing for nasal breathing and nasal resonance. Multiple procedures have been described. [8]
Partial obstruction, either temporary or permanent, of the velopharyngeal port is the unifying feature of most current operative management strategies for velopharyngeal dysfunction (VPD). The choice between the 2 broad categories of options for VPD depends on the patient’s specific diagnosis. Studies indicate that the success of repair depends on selecting the appropriate procedure according to the anatomy and the movement of the velopharyngeal port.
The first option category consists of (1) lengthening the palate by retropositioning the velum, which can be achieved with a V-Y pushback procedure, an intravelar veloplasty, [9] or a double-opposing Z-plasty; [10] and (2) palatal re-repair. [11]
The second option category involves reduction of the static opening between the nasal and oral pharynges, [12, 13] which is considered a velopharyngeal-narrowing procedure. This narrowing may be accomplished either with a pharyngeal flap or with sphincter pharyngoplasty. The pharyngeal flap creates a single subtotal central obstruction of the velopharyngeal port, leaving 2 open ports laterally. Sphincter pharyngoplasty diminishes the cross-sectional area of the central port.
Sufficient prospective and randomized studies have not been performed on the various procedures for repairing a velopharyngeal port. A coordinated multicenter study that correlates the outcomes for the types of procedures on the basis of velopharyngeal anatomy is necessary. Such a study must be designed to produce data that can be appropriately interpreted, generalized, and applied by surgeons in making definitive conclusions on the optimal procedure for each patient.
Pharyngeal flap
The pharyngeal flap has been the most common method for secondary management of VPD for the past 3 decades. It was originally described in 1876 [14] and was widely adopted in the 1950s. Tissue from the posterior pharyngeal wall is attached to the soft palate, creating a midline subtotal obstruction of the oral and nasal cavities with 2 small lateral openings, or ports (see the images below). Ideally, these lateral openings remain patent during respiration and nasal consonant production and close for oral consonants.
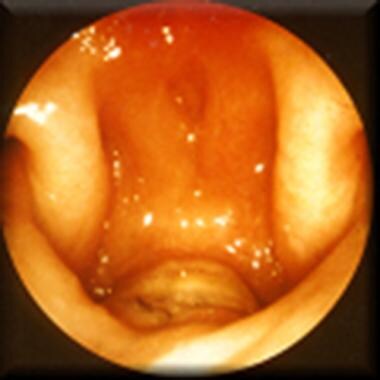 Postoperative nasoendoscopic view of velopharynx, indicating open pharyngeal flap as central subtotal midline obstruction; 2 patent velopharyngeal ports are visible laterally.
Postoperative nasoendoscopic view of velopharynx, indicating open pharyngeal flap as central subtotal midline obstruction; 2 patent velopharyngeal ports are visible laterally.
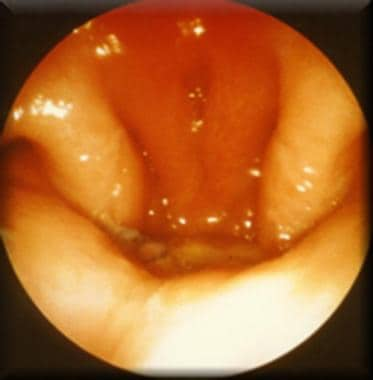 Postoperative nasoendoscopic view of velopharynx, indicating 2 lateral pharyngeal walls opposed to pharyngeal flap to effect complete velopharyngeal closure.
Postoperative nasoendoscopic view of velopharynx, indicating 2 lateral pharyngeal walls opposed to pharyngeal flap to effect complete velopharyngeal closure.
Lateral wall motion is important for effective valving after pharyngeal flap surgery. [15] The procedure seems to benefit patients with satisfactory lateral pharyngeal wall motion with sagittal or circular closure patterns who have a residual velopharyngeal gap of moderate size.
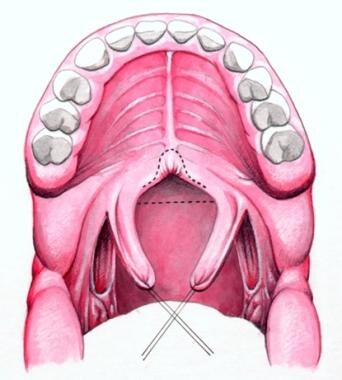
The soft palate is incised in the sagittal midline from the uvula toward the junction of the soft and hard palate. The superiorly based pharyngeal flap is elevated off the prevertebral fascia. The flap is inset to the soft palate and sutured to the nasal side of the soft palate with interrupted sutures. The donor site is partially closed with 3-0 Vicryl sutures.
Nasopharyngeal airways are placed through each lateral pharyngeal port for sizing and postoperative airway support. With the flap inset and the nasal side closed, the soft palate musculature is further dissected and approximated as indicated. The oral side of the soft palate is then closed with interrupted sutures (see the image and the videos below).
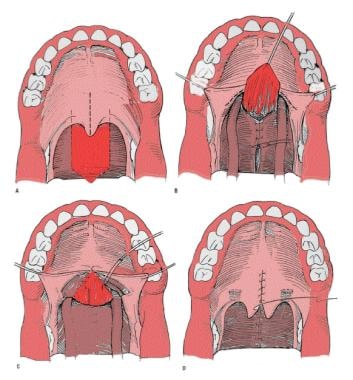 Pharyngeal flap. A mucosal flap from the posterior pharyngeal wall is attached to the soft palate, creating a midline subtotal obstruction of the oral and nasal cavities with 2 small lateral openings, or ports, that ideally remain patent during respiration and nasal consonant production and close for oral consonants.
Pharyngeal flap. A mucosal flap from the posterior pharyngeal wall is attached to the soft palate, creating a midline subtotal obstruction of the oral and nasal cavities with 2 small lateral openings, or ports, that ideally remain patent during respiration and nasal consonant production and close for oral consonants.
The pharyngeal flap procedure may be broken down into steps as follows (see the images below).
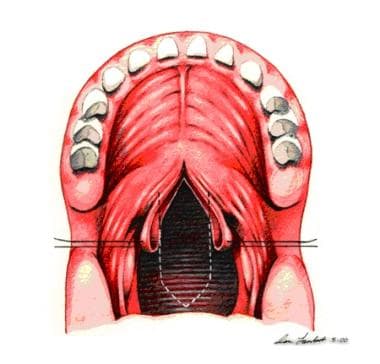
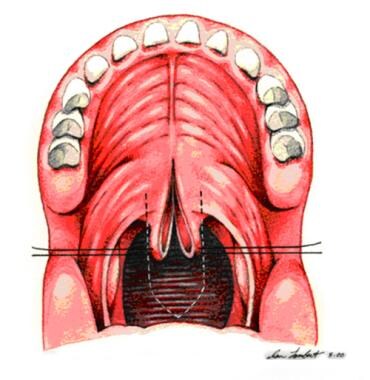 Sutures are placed bilaterally in soft palate to enhance visualization. Midline incision divides soft palate to posterior nasal spine.
Sutures are placed bilaterally in soft palate to enhance visualization. Midline incision divides soft palate to posterior nasal spine.
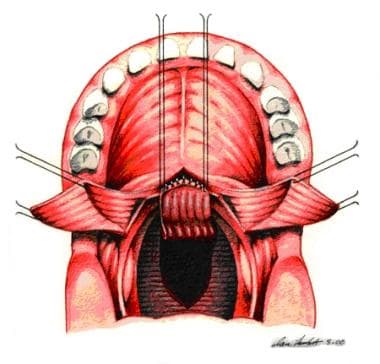
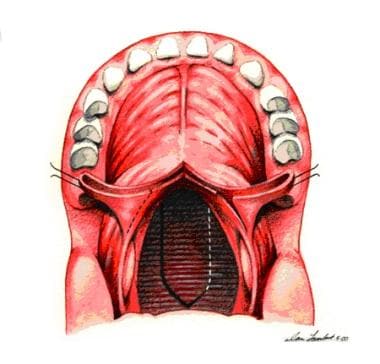 Incision is made along dotted line on posterior pharyngeal wall down to prevertebral fascia. A pharyngeal flap is created. A "book flap" incision that will line lateral ports with mucous membrane is then made bilaterally on nasal surface of soft palate.
Incision is made along dotted line on posterior pharyngeal wall down to prevertebral fascia. A pharyngeal flap is created. A "book flap" incision that will line lateral ports with mucous membrane is then made bilaterally on nasal surface of soft palate.
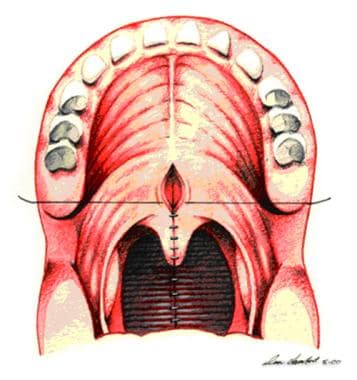
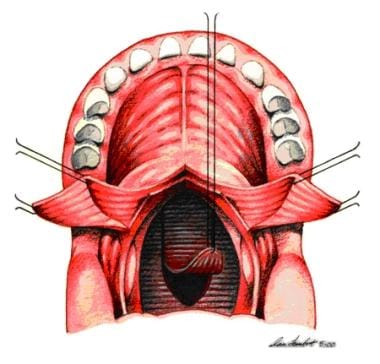 Pharyngeal flap is plotted with indelible ink and elevated to prevertebral fascia. Two soft palate flaps are opened laterally.
Pharyngeal flap is plotted with indelible ink and elevated to prevertebral fascia. Two soft palate flaps are opened laterally.
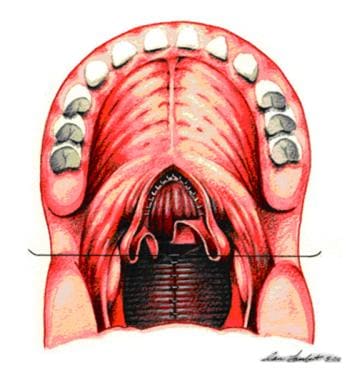 Sutures are placed between pharyngeal flap and nasal edges of soft palate. Raw surfaces arising from origin of pharyngeal flap are closed by simple approximation of tissue.
Sutures are placed between pharyngeal flap and nasal edges of soft palate. Raw surfaces arising from origin of pharyngeal flap are closed by simple approximation of tissue.
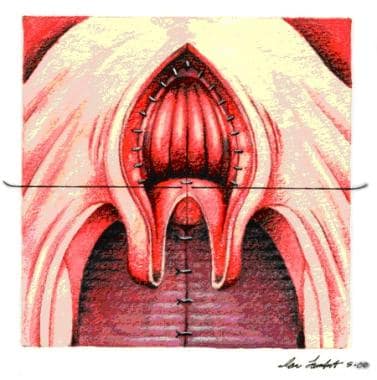 Two flaps from soft palate used to cover raw tissue of pharyngeal flap are sutured to base of pharyngeal flap.
Two flaps from soft palate used to cover raw tissue of pharyngeal flap are sutured to base of pharyngeal flap.
Through the years, several problems and complications have been identified with the pharyngeal flap procedure. As a result, it has undergone several modifications, and variations in specific techniques abound. Key issues that stimulated the development of these modifications include (1) the appropriate width of the flap, (2) whether a superiorly or an inferiorly based flap is more effective in achieving the ideal outcome, and (3) whether the flap should be lined with mucous membrane to prevent postoperative contraction or attenuation of the flap.
The level of flap insertion is linked to surgical success. Insertion of a short, wide flap along the free margin of the soft palate may reduce the contraction of unlined flaps. Placing the flap at this level theoretically narrows the gaps between the base of the flap and the attached tonsillar folds where they merge with the pharyngeal wall. Presumably, this creates a velopharynx that is nearly completely obstructed and requires little contribution of movement from the lateral pharyngeal walls to achieve closure.
Flap width may influence proper closure of the new lateral ports during speech. An excessively wide, nearly obstructive flap may induce untoward consequences (eg, mouth breathing, hyponasality, sleep disturbances ranging from snoring to sleep apnea, or retention of nasal secretions and mucus). If the flap is too long and thin, hypernasality may persist.
Typically, flap width is determined at the time of surgery on the basis of the surgeon’s experience or preference. Many surgeons try to make the flap as wide as the field allows. However, it remains unclear whether appropriate flap width can be routinely determined intraoperatively.
In most cases of postpalatoplasty VPD, control of the flap width based on the morphology observed during the operation is ineffective. However, in cases of gross asymmetric closure patterns, focusing on correcting that asymmetry seems logical. [16, 17] For example, patients with VPD secondary to hemifacial microsomia, stroke, or tumor resection may need specific skewing (tailoring) of flaps to affect closure.
Evidence of significant differences between inferiorly and superiorly based flaps is scant. [18] Currently, most surgeons favor a superiorly based flap. The disadvantages of an inferiorly based flap include length limitation and inferior tethering of the flap below the palatal plane and in the opposite direction of the motion need to effect velopharyngeal closure. [19] Extrapolation from data on failed sphincter pharyngoplasties, in which low flap placement correlated with failure, suggests that a superiorly based pharyngeal flap is preferable. [20]
If the flap is unlined, a broad, raw surface of pharyngeal tissue is left exposed after its elevation. Subsequent contraction (healing by secondary intention of unfulfilled mucosa) may diminish its efficacy. Thus, initial postoperative results may indicate improvement in velopharyngeal function, but symptoms of VPD may recur gradually thereafter. To reduce the tendency for contraction, so-called book flap linings are usually raised from the nasal surface of the posterior velum and folded over to cover the unfulfilled surface of the flap.
A higher surgical success rate can probably be achieved by taking into account an individual patient’s pattern of VPD. How to precisely tailor the flap to balance speech and airway is patient-dependent and objectively difficult to elucidate.
Another modification of the pharyngeal flap is the so-called lined pull-through procedure. [21] This involves demucosalization of the oral surface of the posterior soft palate that juxtaposes with the raw surface of the elevated pharyngeal flap. This probably is not a sound operation, because experience suggests that it results in substantial downward migration/tethering and antagonizes normal velopharyngeal movement.
A surgical technique for modulating the postoperative port size was devised by Hogan in the 1970s. [22] Hogan introduced the concept of lateral port control, using indirect information on the size of the velopharyngeal port derived from differential nasal and oral airflow. This concept was supported by studies done in the 1960s by Warren [23] and Warren and Devereau [24] , which demonstrated that port size is related to the perception of nasal resonance. In 2003, Kummer et al developed the concept further. [25]
Hogan’s technique involves placement of 10-mm2 catheters, which he assumed to be the crucial variable for anticipated normal resonance. [22] Although this technique may seem intuitive and logical, other uncontrolled variables, such as the vagaries of wound healing, scarring, and postoperative migration of the flap, lead the author to believe that port size cannot always be rigorously and reliably controlled.
Sphincter pharyngoplasty
Patients with poor posterior pharyngeal wall movement and smaller velopharyngeal ports tend to benefit more from sphincter pharyngoplasty. The original concept of the procedure was described by Hynes more than 50 years ago [26] ; it has since been modified by others, including Orticochea. [27] Only recently, however, has sphincter pharyngoplasty become the procedure of choice among many surgeons.
The goal of the procedure is to narrow the central velopharyngeal orifice, thus minimizing airflow through the nose during speech. It accomplishes this by rearranging palatopharyngeus myomucosal flaps raised from the posterior tonsillar pillars, which are transposed to the posterior pharyngeal wall and to each other (see the image below). Theoretically, this procedure tightens the central orifice without creating lateral ports, resulting in a velopharyngeal configuration that is the opposite of that achieved with the pharyngeal flap.
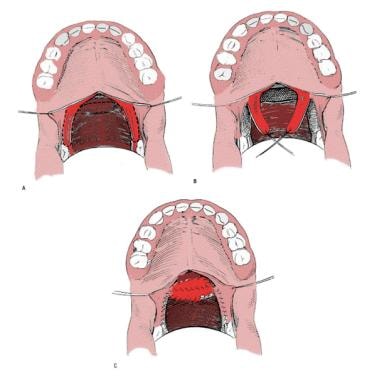 Sphincter pharyngoplasty. The palatopharyngeus muscles are incised bilaterally, and 2 flaps are constructed from the posterior tonsillar pillars. These superiorly based musculomucosal flaps are approximated on the midline of the posterior pharyngeal wall.
Sphincter pharyngoplasty. The palatopharyngeus muscles are incised bilaterally, and 2 flaps are constructed from the posterior tonsillar pillars. These superiorly based musculomucosal flaps are approximated on the midline of the posterior pharyngeal wall.
Sphincter pharyngoplasty may result in less airway morbidity than the pharyngeal flap and may be (at least in theory) more physiologic, although these impressions remain unproven. [28] Because of insufficient collation of data, a detailed description of risks, benefits, and long-term outcomes has not been confirmed.
Sphincter pharyngoplasty may be an appropriate management option for patients with VPD who would not be treated with speech therapy alone and whose nasoendoscopy evaluations indicate a large-gap, coronal, circular, or bow-tie pattern of closure. Essentially, patients who demonstrate good velar elevation but poor lateral wall motion are good candidates for sphincter pharyngoplasty.
The procedure may be divided into steps as follows. First, pass a red rubber catheter transnasally, and suture it to the uvula (see the image below). Reflect the velum into the nasopharynx to achieve exposure of the posterior pharyngeal wall. Inspect the posterior pharyngeal wall for pulsations of aberrant carotid arteries.
Next, plot lines of incision with indelible ink on both the anterior and, with the aid of a retractor, the posterior aspects of the posterior tonsillar pillars, identifying the proposed myomucosal flaps (see the image below). Infiltrate local anesthetic for hemostatic purposes.
Beginning on the right and then repeating the same maneuver on the left, raise the posterior tonsillar pillar as a myomucosal flap, based cephalad (see the image below). Elevate lateral palatopharyngeus myomucosal flaps to the height of attempted velopharyngeal closure, as documented on the preoperative speech videofluoroscopy images.
Incise the posterior pharyngeal wall transversely at the proposed area of insertion in conjunction with the cephalad extent of the elevation of the flaps. The continuous cut extends from the superior end of the posterior limb of one lateral flap to the other flap and allows the lateral flaps to be fully inset. This design eliminates the bilateral fistulae inherent in Orticochea’s original construction. Lay all sutures in sequence and subsequently secure from cephalad to caudad. Remove the red rubber catheter before securing knots.
Attach the superior mucosa of the left flap to the mucosa of the superior incision of the posterior pharyngeal wall. Attach the caudal mucosa of the left flap to the superior mucosa of the right flap, overlapping the 2 flaps as described by Hynes. Attach the caudal mucosa of the right flap to the inferior mucosa of the posterior pharyngeal wall (see the images below).
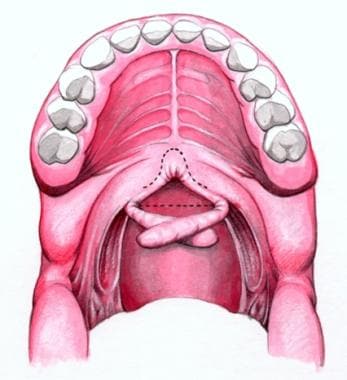 Schematic showing rotation of palatopharyngeal flaps through 90°. It is ready for attachment to posterior pharyngeal wall.
Schematic showing rotation of palatopharyngeal flaps through 90°. It is ready for attachment to posterior pharyngeal wall.
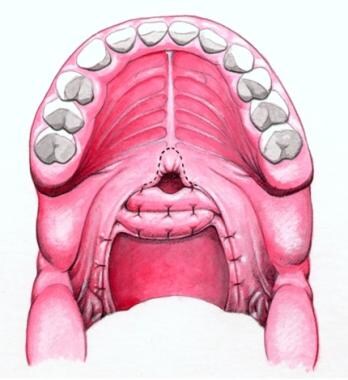 Schematic showing completed sphincter pharyngoplasty. Flaps are overlapped and are sutured to each other and to posterior pharyngeal wall.
Schematic showing completed sphincter pharyngoplasty. Flaps are overlapped and are sutured to each other and to posterior pharyngeal wall.
Assist the integrity of the newly created sphincter by suturing the lateral flaps securely to one another and to the superior constrictor and pharyngobasilar membrane. Attempt to capture the mucosa, submucosa, and epimysium with each stitch to maximize the suture’s holding power. Approximate tissues without tension, and close the donor sites. Suture with 4-0 polyglactin. After construction of the sphincter pharyngoplasty, place an orogastric tube, aspirate gastric contents, and remove the tube.
The central orifice of the sphincter pharyngoplasty port at the conclusion of the procedure should be approximately a small fingerbreadth in width (approximately 1 cm in diameter). A tight sphincter pharyngoplasty port usually measures approximately 0.5 cm in diameter, and a loose sphincter pharyngoplasty port usually measures approximately 1.5 cm in diameter.
Riski et al demonstrated that the height of insertion appears to be a critical factor for success in sphincter pharyngoplasty. They documented the emphasis on the importance of inset height for placement of the myomucosal flaps. [20]
In a follow-up study, Riski et al reported results in a large number of patients over a 15-year span. [29] Results showed a high success rate among patients who underwent sphincter pharyngoplasty before speech dysfunction developed fully. Success also seemed to correlate with a patient age of less than 6 years at the time of operation.
In a study in which preoperative speech and instrumental assessments were separated to provide perceptual information and physiologic relationships, Witt et al reported that only 18% of the patients showed 100% resolution of hypernasality and nasal emission. [30] Approximately 30% developed hyponasality or obstructed speech and breathing patterns. Sphincter pharyngoplasty remains an effective treatment modality for VPD; however, the study does emphasize the need for further comparative data.
Palatal lengthening
The primary goals of this procedure are to lengthen the palate, occlude a small gap in the velopharyngeal port, and retrodisplace the palate in a more physiologically normal place. The most commonly used procedure for palatal lengthening is known as the V-Y pushback procedure (Veau-Wardill-Kilner operation). It is designed to displace the palatal mucoperiosteum and velar musculature after a primary palatoplasty has been performed.
Because this procedure does not obstruct the nasal airway, velopharyngeal valving may be achieved without the troublesome adverse effects of complete nasal obstruction (eg, chronic mouth breathing, sleep apnea). However, numerous problems are reported in association with its use. The extensive mucoperiosteal stripping the technique requires may have a deleterious effect on long-term facial growth. High rates of fistulization have been reported, and the ultimate length gain with this surgical maneuver is unpredictable.
During the past 20 years, the Furlow double-opposing Z-plasty palatoplasty has become accepted as a means of gaining palatal length and restoring the velar musculature anatomically. [31, 32, 33, 34, 35, 36] It provides for closure of the hard and soft palates in a single procedure without pushback or lateral relaxing incisions. Modifications of this procedure from the Children’s Hospital of Philadelphia include lateral relaxing incisions.
The unique repair of the soft palate is achieved with mirror-image musculomucosal Z-plasties to retroposition and overlap the soft palate muscles to recreate the palatal muscular sling. The Z-plasties provide length to the velum without borrowing tissue from the hard palate.
Posterior pharyngeal wall augmentation
A wide variety of implantable materials have been used to augment the posterior wall. Problems with extrusion, migration, resorption, and infection have been reported. Silicone has been noted to have a high extrusion rate. Teflon is not advised for use in the pharynx due to concerns over the risk of injection into large blood vessels. Both silicone and Teflon also have a tendency to migrate inferiorly along the prevertebral fascia. Autologous tissue, such as fat or rolled dermis, has been less problematic but tends to resorb with time.
More recent reports have described the use of calcium hydroxyapatite injected into the posterior pharyngeal wall. This technique has been demonstrated to have a reasonably high success rate with no migration or extrusion of the injected material. Polyethylene implants imbedded into the posterior pharynx has also demonstrated success in some adult patients.
For any of these procedures, suturing is performed with 3-0 or 4-0 Vicryl sutures because chromic sutures tend not to provide sufficient holding strength and may result in repair dehiscence. Mucosal closure may be performed with chromic sutures as long as closure tension is not too great.
Autogenous posterior pharyngeal wall augmentation is used in various centers around the world, particularly in patients with small velopharyngeal gaps; varying degrees of success have been reported. Though accepted in Europe and Russia, autogenous posterior pharyngeal wall augmentation has not gained widespread acceptance in North America.
The operative technique involves raising a U-shaped posterior pharyngeal wall myomucosal flap to the tubercle of the atlas. The tip of the flap is connected by deep bites in the epimysium to the base of the flap. By securing the knots, the flap is rolled on itself, creating a soft tissue bulge or augmentation.
Outcome data are scant. In one report, results of extramural judgments showed no statistically significant tendency for patients’ speech to be rated as more normal after the augmentation procedure than before it. [37]
This study involved recorded (audio/video) perceptual, nasoendoscopic, and fluoroscopic standardized speech and airway evaluations, comparing preoperative and postoperative assessments. [37] A randomized master tape was presented in blinded fashion and random order to 3 skilled raters for independent assessment of numerous perceptual and instrumental parameters of speech. The raters were uninvolved in the care of the patients, and their intraobserver and interobserver reliabilities were known.
Dermal grafting for fistula closure
Palatal fistulas are problematic for hygiene and speech. The contribution of fistulas to VPD requires multidisciplinary assessment.
Recent lines of clinical and experimental evidence support the efficacy of decellularized dermal grafting as an adjunct to the closure of recurrent oronasal fistulas. [38, 39, 40] Early results with decellularized dermal grafts, placed between the nasal mucosa and the levator veli palatine muscle, appear promising at decreasing the rates of infection, dehiscence, signs of rejection, extrusion, and fistula recurrence. All patients were followed for an average of 3 months. [40]
These authors have concluded that decellularized dermal graft should be considered for use in the treatment of recurrent oronasal fistula after cleft palate repair. [41] Obviously, further clinical study is needed. This novel method offers promise as a simple and effective technique for tension-free closure of oronasal fistulas.
Post-Procedure
Postoperative care
Patients are monitored overnight with pulse oximetry and are given oxygen via a nasal cannula. They may resume a soft or liquid diet immediately. Most patients are discharged from the hospital after 1 night, although patients with 22q11 deletion often require at least 2 nights in the hospital. Patients are instructed to sleep with their heads elevated on several pillows for 2 weeks. Oral hygiene is encouraged.
Patients are seen 3 weeks after surgery for a follow-up evaluation. In the meantime, parents are given information about sleep apnea and breathing signs that may be indicative of a problem. The team nurse communicates with parents by telephone, and a highly integrated and focused program of speech therapy usually resumes 3-6 weeks after surgery for VPD intervention.
Postsurgical velopharyngeal assessment is performed at 3 and 12 months postoperatively and consists of the same perceptual, nasoendoscopic, fluoroscopic, and airway evaluations performed preoperatively. Follow-up with a speech pathologist is essential to continue reinforcement of correct speech and to help prevent residual articulation errors.
Outcome and prognosis
Studies have shown success rates for pharyngeal flap surgery of 80-90%. The classification of failure or success depends on the investigator. Certain studies classify patients with hyponasality as a successful outcome. Other studies classify postsurgical hyponasality of greater than 4 years a failure. In those studies, the success rate is somewhat lower.
Sphincter pharyngoplasty has been associated with lower success rates of 40-60%. [42, 43] More recent studies show that the success rate can reach approximately 80% with appropriate patient selection based on velopharyngeal port anatomy.
The effectiveness of the Furlow procedure in properly selected patients is reported to be greater than 90% in most series. Ongoing debate in the literature surrounds patient selection for the Furlow procedure. [44] However, newer studies continue to demonstrate its success as a method for speech salvage after failed palate repair. [45]
Some surgeons have attempted to improve success rates by a combination of techniques. One study demonstrated that combination of a Furlow palatoplasty with the sphincter pharyngoplasty had better outcomes than the sphincter pharyngoplasty alone. [46]
Complications
Risks involved with surgical VPD treatment include sleep disturbances (ranging from simple snoring to acute obstructive sleep apnea), dehiscence (procedure failure), and potential iatrogenic injury to anomalous internal carotid arteries. [47, 48]
Rarely is sleep apnea severe enough to necessitate hospitalization. This adverse effect appears to occur in a substantial percentage of patients surgically managed for VPD, as suggested in a preliminary report by Witt et al that cited an incidence of 13% in the 58 patients observed. [28]
A history of perinatal respiratory dysfunction, early age at sphincter pharyngoplasty, upper respiratory tract infections, and microretrognathia are risk factors for acute obstructive sleep apnea. The severity of this complication diminishes with time, partially because of a reduction in edema and postoperative inflammation.
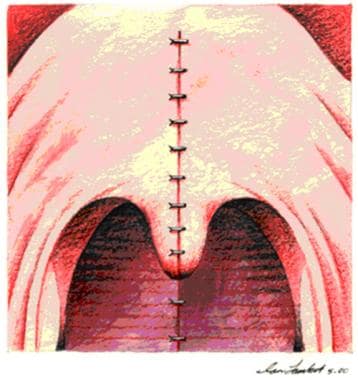
Complete nasopharyngeal obstruction (see the image below) should be a rare complication, provided that all raw surfaces were properly fulfilled at the primary pharyngoplasty.
Dehiscence correlates inversely with surgical experience and directly with a previous history of tonsillectomy or adenoidectomy. Either of these procedures may result in compromised blood flow to the palatopharyngeal flaps during performance of a sphincter pharyngoplasty.
Persistent hypernasality usually results from a port size that is too large. Hyponasality is caused by constriction of the port, overtightening of the port, or closure from contracture or scar formation.
Velopharyngeal surgery is still more an art than a science. The goal is to create a subtotal obstruction that improves resonance but avoids airway morbidity. Still, in approximately 10% of cases, reoperation is necessary to treat residual hypernasality or nasal emission.
-
Sphincter pharyngoplasty. The palatopharyngeus muscles are incised bilaterally, and 2 flaps are constructed from the posterior tonsillar pillars. These superiorly based musculomucosal flaps are approximated on the midline of the posterior pharyngeal wall.
-
Pharyngeal flap. A mucosal flap from the posterior pharyngeal wall is attached to the soft palate, creating a midline subtotal obstruction of the oral and nasal cavities with 2 small lateral openings, or ports, that ideally remain patent during respiration and nasal consonant production and close for oral consonants.
-
Coronal closure.
-
Sagittal closure.
-
Circular closure.
-
Circular closure with Passavant's ridge.
-
Patient with severe articulation disorder and velocardiofacial syndrome. Little or no velar closure is noted on nasopharyngoscopy, known as a "black hole." Surgical treatment is with a wide pharyngeal flap. Aberrant carotid arteries coursing through the nasopharynx complicate surgical management.
-
Schematic lateral view of velopharynx, illustrating anatomy.
-
Schematic bird's-eye view of velopharynx, illustrating directional movements of representative closure patterns.
-
Palatal lift. Hard and soft palatal components are shown.
-
Palatal lift in situ.
-
Preoperative nasoendoscopic view of velopharynx, showing lateral pharyngeal wall (1, 2), velum (3), and tonsil (4).
-
Postoperative nasoendoscopic view of velopharynx, indicating open pharyngeal flap as central subtotal midline obstruction; 2 patent velopharyngeal ports are visible laterally.
-
Postoperative nasoendoscopic view of velopharynx, indicating 2 lateral pharyngeal walls opposed to pharyngeal flap to effect complete velopharyngeal closure.
-
Sutures are placed bilaterally in soft palate to enhance visualization. Midline incision divides soft palate to posterior nasal spine.
-
Soft palate flaps are retracted.
-
Incision is made along dotted line on posterior pharyngeal wall down to prevertebral fascia. A pharyngeal flap is created. A "book flap" incision that will line lateral ports with mucous membrane is then made bilaterally on nasal surface of soft palate.
-
Pharyngeal flap is plotted with indelible ink and elevated to prevertebral fascia. Two soft palate flaps are opened laterally.
-
Free inferior edge of pharyngeal flap is sutured to posterior edge of soft palate.
-
Sutures are placed between pharyngeal flap and nasal edges of soft palate. Raw surfaces arising from origin of pharyngeal flap are closed by simple approximation of tissue.
-
Two flaps from soft palate used to cover raw tissue of pharyngeal flap are sutured to base of pharyngeal flap.
-
Oral side of soft palate is sealed to conceal pharyngeal flap.
-
Immediate postoperative view from oral cavity.
-
Schematic of lateral view. Catheter has been passed transnasally and attached to uvula.
-
Schematic showing proposed incisions (dashed lines).
-
Schematic showing elevation of both tonsillar pillar flaps.
-
Schematic showing rotation of palatopharyngeal flaps through 90°. It is ready for attachment to posterior pharyngeal wall.
-
Schematic showing completed sphincter pharyngoplasty. Flaps are overlapped and are sutured to each other and to posterior pharyngeal wall.
-
Complete nasopharyngeal stenosis.
-
Hard and soft palates. A: transverse rugae of hard palate; B: median raphe of hard palate; C: median raphe of soft palate.