Background
Submuscular breast augmentation involves placement of an implant in a plane below the pectoralis major muscle. Breast augmentation is usually performed to enhance the size and shape of a woman's breasts. Surgery can often improve an individual's self-image. Historically, breast enlargement has been accomplished by one of four methods, with varying degrees of success.
-
Inert materials such as silicone or paraffin have been injected directly into the parenchymal tissue to increase breast size. This method was abandoned because of the exceedingly high incidence of acute and long-term complications. Granuloma formation frequently occurs with this procedure, as well as skin loss and scar contracture, producing disfigurement.
-
Autogenous tissue injections also have been used in an attempt to enhance and enlarge the breast shape. Autogenous tissues, including omentum, fat, muscle, lipomas, and skin, in the form of dermis or dermal fat grafts, have been employed to enhance and enlarge the breasts. Results from injection of autogenous tissue have lacked predictability. In addition to the risks of scarring and uneven texture that may be visible in patients who have undergone tissue injection into the breasts, microcalcifications may develop. This makes performing follow-up mammography on these women for early diagnosis of breast cancer difficult.
-
A suction pump device has been used to attempt to enhance the breast shape. However, while some enlargement was noted, the overall aesthetic shape in these individuals was lacking significantly.
-
Implants filled with a substance can be placed to enhance and enlarge the size of the breast.
Implants, most often filled with either silicone or saline, have been used since the 1960s to enhance and enlarge the shape of female breast tissue. Currently, they are the preferred method for augmentation mammaplasty. [1]
Using data from the American Board of Plastic Surgery, Stein et al reported that among aesthetic primary breast implant surgeries, the rate of submuscular augmentations increased between 2005 and 2021, while the rate of subglandular and dual-plane augmentations decreased. Between 2005 and 2014, the submuscular procedure rate was 22%, with that figure increasing between 2015 and 2021 to 56%. For subglandular augmentation, however, the rates for the two time periods were 19% and 7%, respectively, and for dual-plane augmentation they were 57% and 35%, respectively. (The investigators suggested, though, that some surgeons, in specifying submuscular procedures, may have actually been referring to dual-plane operations.) [2]
History of the Procedure
Both saline-filled and silicone-filled implants have been used since the 1960s to enhance and enlarge women's breasts. Initial silicone implants in the early 1960s had a thick elastomer shell and were filled with a relatively firm silicone gel. In the mid 1970s, silicone implants tended to have a thin elastomeric shell and a less viscous gel, though gel cohesiveness was varied. Over the years, modifications have been made to implant shape, shell texture and properties, and the substance with which the implant is filled.
Implant shape
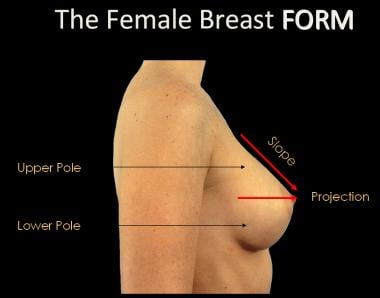
Implants can be round or teardrop-shaped. Round implants are disc-shaped and exhibit equal fullness in all 4 quadrants of the breast (see the image below). Round implants come in a variety of projections, with the different projections affecting the final shape of the breast. Projections include moderate profile, moderate plus profile, high profile, and ultrahigh profile. The greater the height of the implant (profile), the narrower the base diameter. Higher-profile implants provide more upper pole fullness to the breast and greater lift.
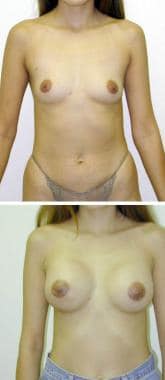 (Above) Preoperative view of 28-year-old woman with micromastia. She has had 2 children. Note the small breast has decreased upper pole fullness. (Below) Postoperative view after submuscular augmentation with a round implant. Notice increased fullness of the upper poles of the breasts. Submuscular placement makes it difficult to appreciate the edge of the implant.
(Above) Preoperative view of 28-year-old woman with micromastia. She has had 2 children. Note the small breast has decreased upper pole fullness. (Below) Postoperative view after submuscular augmentation with a round implant. Notice increased fullness of the upper poles of the breasts. Submuscular placement makes it difficult to appreciate the edge of the implant.
Teardrop-shaped implants (also called anatomic implants) exhibit reduced augmentation fullness in the upper pole of the breast and increase fullness in the lower half of the breast (see the image below). These implants are narrower than rounded implants at the superior and inferior poles. In some cases, implant rotation can be a problem with teardrop-shaped implants.
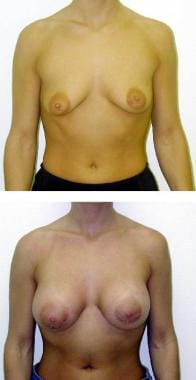 (Above) Preoperative view of 26-year-old woman with minimal upper pole fullness and asymmetric breasts. (Below) Postoperative result after submuscular placement of anatomically shaped implants. Notice increased lower pole fullness and absence of upper pole fullness relative to the round implant.
(Above) Preoperative view of 26-year-old woman with minimal upper pole fullness and asymmetric breasts. (Below) Postoperative result after submuscular placement of anatomically shaped implants. Notice increased lower pole fullness and absence of upper pole fullness relative to the round implant.
Currently available devices in the United States are saline- or silicone-filled implants with either textured or smooth surfaces (see the subsection below). Companies with US Food and Drug Administration (FDA) approval to market these devices in the United States include Allergan Aesthetics, Ideal Implant Inc, Mentor Worldwide LLC, and Sientra Inc. [3]
Shell texture and properties
Polyurethane-covered silicone gel implants were first introduced in the early 1970s. Initial reports indicated that polyurethane-covered silicone gel implants resulted in decreased capsule formation. Ingrowth of scar tissue into the polyurethane surface was postulated to break up the vector forces of scar contracture. Because of the altered vectors of scar contracture, the capsule of the scar was unable to contract to the same degree as typically present around a silicone implant; however, this theory never was proven definitively in a scientific study. [4, 5]
Over time, however, polyurethane underwent microfragmentation and phagocytosis. In addition, the polyurethane could break down and dissolve within the local tissue after implant placement. [6] An intense foreign-body reaction with numerous macrophages and multinucleated giant cells occurred in the capsule in some patients who had this type of implant.
Because of these problems, polyurethane-covered implants were removed from the market. In an attempt to duplicate polyurethane’s potential protection against capsular contracture, implant manufacturers increased the shell thickness of standard saline and silicone implants with surface texturing. Unfortunately, shell texturing has not conclusively decreased capsular contractures in patients undergoing breast augmentation. Increased wrinkling due to textured implants has been reported.
In the late 1980s and into the 1990s, multi-layered shells were developed. These have a barrier layer to reduce silicone leakage from the implant. The alteration in the elastomer shell composition was accompanied by the introduction of a firmer gel. The firmer gel has multiple crosslinks between the silicone molecules to obtain a cohesive effect, which reduces the capacity of the gel to migrate significantly if the integrity of the shell is compromised.
Silicone vs saline
Silicone implants were first used in 1964, when reported by Cronin and Gerow, and they are still used for breast augmentation today. [7] The advantages of silicone-filled implants include the minimal solubility of silicone and favorable viscosity of the material, which, together, provide a natural feel. The initial silicone implants were composed of a thick outer elastomer shell that was then filled to variable volumes with a silicone gel. Problems associated with silicone implants include capsular contracture, granulomas that develop following leaching of silicone from the implant, and migration of silicone into the axilla. In 1992, the FDA temporarily removed silicone implants because of a purported increased incidence of autoimmune phenomena.
Saline implants had also been in use since the 1960s, but the removal of silicone implants from the market in 1992 increased interest in saline implants. The advantages of saline-filled implants are that saline poses no risk to patients and is safely absorbed into the bloodstream if the integrity of implant capsule is compromised. Some studies report that saline implants have a decreased capsular contracture rate compared to silicone implants. However, saline has slightly decreased viscosity compared to silicone. The initial use of saline implants has resulted in a high incidence of deflation (approximately 10%). Over the last 10 years, implant manufacturers have significantly improved the integrity of implants; specifically, the reliability of the valvular mechanism for introduction of saline into the implant. Late deflation of saline-filled implants, however, remains a significant issue.
After having been removed from general use by the FDA, silicone implants were exhaustively studied and have been shown to have low complication rates comparable with or, in some cases, lower than those of saline implants (see discussion below). The reported autoimmune responses were discredited by numerous exhaustive long-term studies that failed to demonstrate increased incidence of long-term problems in large numbers of women who underwent augmentation with silicone implants.
In 1999, the National Academy Institute of Medicine issued a report that concluded "because there are more than 1.5 million adult women of all ages in the United States with silicone breast implants, some of these women would be expected to develop connective tissue diseases, cancer, neurological diseases, or other systemic complaints or conditions. Evidence suggests that such diseases or conditions are no more common in women with breast implants than in women without implants." [8]
Women in the United States have an approximately 13% average risk of developing breast cancer at some time in the lives. [9] A 2000 study from the National Cancer Institute involving over 13,000 women concluded that there was no significant increase in breast cancer incidence in women who underwent breast augmentation, no matter the type of implant used. [10] However, anaplastic large T-cell lymphoma of the breast has since been identified as having a potential, albeit rare, association with breast implants. [11, 12, 13] (See Complications.)
Patients who have undergone breast augmentation and develop breast cancer do not experience delayed detection or poorer survival than the general population. [14, 15]
In 2006, the FDA approved implants filled with silicone gel for augmentation and reconstructive purposes in the United States. [16] Silicone implants were never removed from the European market. For complete information on silicone safety, see the Medscape Drugs & Diseases article Silicone Breast Implant Safety and Efficacy. Implant companies now offer altered types of silicone gel with increased cohesive properties.
Relevant Anatomy
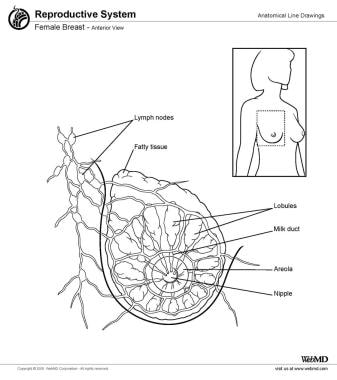
The breast is made up of fatty tissue and glandular, milk-producing tissues (see the image below). The ratio of fatty tissue to glandular tissue varies among individuals. In addition, with the onset of menopause (ie, decrease in estrogen levels), the relative amount of fatty tissue increases as the glandular tissue diminishes.
The base of the breast overlies the pectoralis major muscle between the second and sixth ribs in the nonptotic state. The gland is anchored to the pectoralis major fascia by the suspensory ligaments first described by Astley Cooper in 1840. These ligaments run throughout the breast tissue parenchyma from the deep fascia beneath the breast and attach to the dermis of the skin. Since they are not taut, they allow for the natural motion of the breast. These ligaments relax with age and time, eventually resulting in breast ptosis. The lower pole of the breast is fuller than the upper pole (see the image below).
For more information about the relevant anatomy, see Breast Anatomy.
Contraindications
Severe ptosis is a relative contraindication to surgery. Women with significant ptosis may require a mastopexy concomitantly or as a secondary procedure.
Women with tubular breasts also are at significant risk of secondary procedures after the augmentation to correct residual deformities.
As with any surgery, severe associated medical conditions need to be evaluated on a patient-by-patient basis.
-
(Above) Preoperative view of 28-year-old woman with micromastia. She has had 2 children. Note the small breast has decreased upper pole fullness. (Below) Postoperative view after submuscular augmentation with a round implant. Notice increased fullness of the upper poles of the breasts. Submuscular placement makes it difficult to appreciate the edge of the implant.
-
(Above) Preoperative view of 26-year-old woman with minimal upper pole fullness and asymmetric breasts. (Below) Postoperative result after submuscular placement of anatomically shaped implants. Notice increased lower pole fullness and absence of upper pole fullness relative to the round implant.
-
(Above) A 24-year-old woman with an A-cup breast. (Below) Postoperative result after subglandular placement with 460-mL implants. Note the lower position of the implant when placed in a subglandular position.
-
(Above) Preoperative view of a 27-year-old patient desiring augmentation. (Below) Postoperative view after augmentation with a round implant. Notice the smooth contour in the upper pole. The end of the implant is not perceptible because of submuscular placement.
-
(Above) Preoperative view of 23-year-old patient with micromastia. (Below) Postoperative view after augmentation with detachment of the inferior half of the pectoralis musculature from the sternal attachments to provide cleavage. Note increased medial projection.
-
Female breast, anterior view.
-
The female breast form.