Background
The management of complex abdominal wall defects has challenged both general surgeons and reconstructive surgeons since the turn of the last century. Formerly the domain of the general surgeon, the increasing complexity of abdominal wall defects and the development of techniques involving manipulation and mobilization of muscle and myocutaneous flaps have drawn on the expertise of the plastic surgeon. Regardless of the surgeon’s background, the goals of the reconstructive surgeon in managing complex abdominal wall defects are to restore the structural and functional continuity of the musculofascial system and to provide stable and durable wound coverage.
Prior to discussing an approach to repair, it is helpful to appreciate the magnitude of the problem posed by incisional hernias and other abdominal wall defects. From 2008-2009, approximately 381,000 cases of incisional hernia repair were reported, with an expected increase in case volume of 1-2% annually. [1] This increase has been attributed to the growing age of the population, increasing rates of obesity and diabetes, improved survival from intra-abdominal cancers, and improvements in care of the critically injured patient, yielding greater survival of patients following abdominal catastrophe. These factors have produced a large subset of medically complicated patients with structurally complex abdominal wall defects.
A study Ortega-Deballon et al found that in France, of 710,074 patients who underwent abdominal surgery between 2013 and 2014, 4.6% (32,633) required at least one incisional hernia repair in the first 5 years post abdominal surgery. A 15.7% recurrence rate was found following repair. [2]
Prompted by the increasing complexity of the ventral hernia patient, interest in understanding the pathophysiology for hernia formation has increased. Likewise, surgical techniques have evolved to emphasize restoration of abdominal wall function as well as structure. Abdominal wall defects are no longer characterized as “holes,” but as chronic wounds that result in a complex neuromuscular deformity. Clinical and animal studies exploring the mechanisms underlying hernia formation have implicated both mechanical and biologic factors. Preclinical studies exploring the biology of hernia formation have demonstrated a preponderance and persistence of type III collagen in laparotomy wounds that go on to develop hernias. While type III collagen is found in the provisional matrix of a healing wound, it is the failure of this type III collagen to be replaced by mature type I collagen that has led many to consider a hernia a “chronic wound.”
Supporting the biologic etiology of hernia formation are preclinical studies that demonstrate divergent genetic patterns displayed by laparotomy wounds that heal and those that herniate. This is also consistent with the recognition of aneurysmal disease and connective-tissue disorders as significant risk factors for hernia formation. [3]
From a mechanical standpoint, greater understanding of the abdominal wall as a dynamic musculotendinous structure has led to characterization of a laparotomy incision within the linea alba as a tendon injury, akin to laceration of an upper extremity tendon. The laparotomy, as tenotomy, leads to myocyte replacement with fibrotic connective tissue and disordered arrangement of sarcomere structure, manifesting as a decrease in abdominal wall compliance that predisposes the abdominal wall to further injury.
In the aforementioned French study by Ortega-Deballon and colleagues, older age was found to be a risk factor for needing incisional hernia repair within 5 years of abdominal surgery, the hazard ratio (HR) being 4.1 for patients aged 56-70 years. Other risk factors included the following [2] :
-
Hospital stay of at least 10 days - HR = 3.1
-
Laparotomy (vs laparoscopy) - HR = 1.9
-
Obesity - HR = 1.9
-
Chronic obstructive pulmonary disease (COPD) - HR = 1.3
-
Male gender - HR = 1.1
Moreover, the risk for needing incisional hernia repair in the Ortega-Deballon study was 1.4 times higher for patients who had a small bowel or peritoneum operation, and 1.2 times higher for those in whom a colorectal procedure was performed, than for individuals who underwent hepatobiliary surgery. [2]
The concept of optimal tension has also been extrapolated from the hand surgery literature and applied to an understanding of hernia biology. The resulting shift from a “tension-free repair” to a repair under “physiologic tension” has led to changes in surgical technique, with a greater preference for lighter-weight prosthetic materials and more widespread use of components separation, a procedure designed to improve abdominal wall compliance.
Indications
The abdominal wall serves to protect the abdominal organs, maintain upright posture and support the spine, and assist in bodily functions that require generation of Valsalva, such as coughing, urination, or defecation. There is also a suggestion that the absence of an intact abdominal wall results in loss of the mechanical endpoint of satiety, leading to unintentional weight gain. Indications for reconstruction can be both symptomatic and structural, with goals ranging from pain relief to prevention of incarceration. That said, while large abdominal wall defects can be plagued by significant herniation of intra-abdominal contents, the size of the fascial defect puts them at low risk for incarceration. Defects that ultimately require reconstruction may stem from trauma, tumor extirpation, previous abdominal procedures, and surgical management of severe infection.
The rate of incisional hernia following celiotomy ranges from 3-20%. Factors associated with the formation of an incisional hernia include wound infection, immunosuppression, malnutrition, morbid obesity, previous abdominal operation, patient age, and medical conditions (eg, prostatism) that may cause an increase in intra-abdominal pressure postoperatively. [4] Other biologic risk factors that have become known in recent years include connective-tissue disorders such as Ehlers-Danlos syndrome, a history or family history of aneurysmal disease, or lathyrism, an acquired inhibition of collagen cross-linking due to a diet high in certain legumes (eg, chick peas). [5]
Of the various benign tumors that develop within the abdominal wall, desmoid tumors are the most common. These lesions are histologically benign but locally invasive. Treatment consists of full-thickness abdominal wall resection. Local recurrence rates remain approximately 40%, and it usually occurs within 2 years, despite aggressive treatment. Adjuvant radiotherapy may be required when margins are inadequate. [6]
Treatment of malignant tumors of the abdominal wall requires aggressive resection of involved skin and subcutaneous tissue, as well as the myofascial component if it is violated by the tumor. Sarcomas are the most common and require both aggressive resection and radiotherapy. Intra-abdominal tumors can also involve the abdominal wall, either from contiguous or hematogenous spread. Reconstruction of the abdominal wall in these cases is usually directed by the extent of resection and the possibility of further surgical intervention (see case example images below).
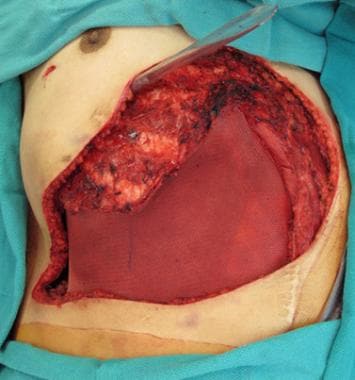
 Forty-six-year-old patient with chest wall recurrence of primary visceral carcinoma requiring composite resection.
Forty-six-year-old patient with chest wall recurrence of primary visceral carcinoma requiring composite resection.
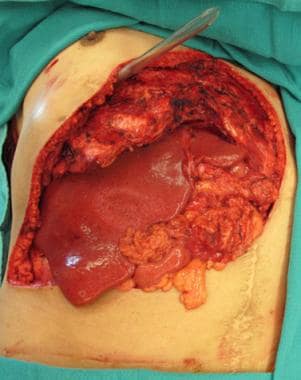 Resultant complex abdominal wall defect following resection. Note repair of the diaphragm to the revised costal margin and exposure of the liver.
Resultant complex abdominal wall defect following resection. Note repair of the diaphragm to the revised costal margin and exposure of the liver.
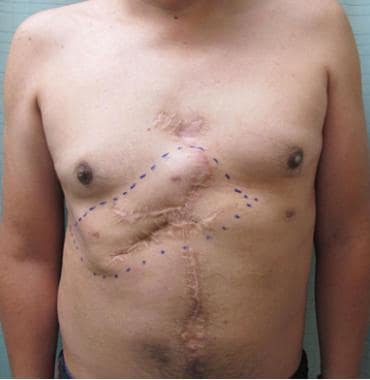
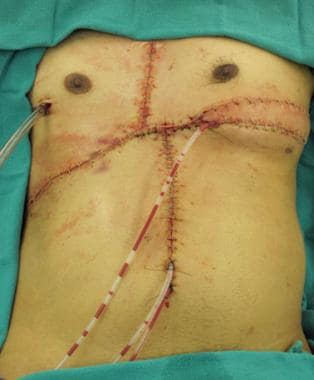 Soft tissue closure of defect with right-sided pectoralis muscle flap, left myocutaneous latissimus flap, and "4-quadrant" fasciocutaneous advancement flaps.
Soft tissue closure of defect with right-sided pectoralis muscle flap, left myocutaneous latissimus flap, and "4-quadrant" fasciocutaneous advancement flaps.
Abdominal wall defects associated with traumatic abdominal injuries are commonly a result of penetrating injury. These abdominal wounds may be grossly contaminated from simultaneous bowel injuries and require delayed reconstruction in multiple stages.
Abdominal wall soft tissue infections are rare except as a complication of a prior mesh repair of abdominal fascia. However, cases of necrotizing fasciitis following inadvertent bowel injury during abdominal liposuction have been reported. Abdominal wall mesh infections commonly present as draining sinuses over the abdomen. Mesh infections are resistant to wound care and antibiotics. Often, successful treatment of the abdominal infection requires removal of the infected mesh and staged abdominal reconstruction. [7]
Regardless of the indication for repair, it is important to have clearly defined goals and objectives when proceeding with abdominal wall reconstruction. In some cases, the complexity or contaminated nature of the case may preclude definitive reconstruction in a single stage. In these situations, it may be prudent to cover or close the wound first, and defer management of the fascial defect until the patient’s status and operative conditions can be optimized. Attempts to treat both the wound and the fascial defect simultaneously may compromise the durability of the fascial repair or unnecessarily predispose the patient to complications such as infection or fistula formation.
Relevant Anatomy
Anterior abdominal wall anatomy
The anatomical layers of the abdominal wall include skin, subcutaneous tissue, superficial fascia, deep fascia, muscle, extraperitoneal fascia, and peritoneum. This anatomy may vary with respect to the different topographic regions of the abdomen. The major source of structural integrity and strength of the abdominal wall is provided by the musculofascial layer. The main paired abdominal muscles include the external oblique muscles, internal oblique muscles, transversus abdominis muscles, and rectus abdominis muscles and their respective aponeuroses, which are interdigitated with each other, and provide core strength and protection to the abdominal wall viscera. The integrity of the abdominal wall is essential not only to protect the visceral structures but also to stabilize the trunk and to aid trunk movement and posture.
Skeletal system
Early 19th century anatomist August Rauber described the large gap in the skeletal system between the lower edges of the thorax and the upper edge of the pelvis as the lacuna sceleti sternopubica. This gap is closed by the abdominal muscles and their aponeuroses. The skeletal system, which is relatively fixed, provides attachment points for the soft tissue and muscles of the abdominal wall. The skeletal anatomy of the abdomen consists of the xiphoid process, the costal cartilages of ribs 7-10, the floating ribs 11 and 12, the L1-L5 vertebrae, the iliac crests, the anterior superior iliac spine (ASIS), the pubic tubercle/pubic crest, and the pubic symphysis. The abdominal wall musculoaponeurotic structure is attached to the ribs superiorly, the bones of the pelvis inferiorly, and the vertebral column posteriorly.
Superficial fascia
The superficial fascia of the abdominal wall is divided into a superficial and a deep layer. It may be as thin as half an inch or less or as thick as 6 inches or more. Above the umbilicus, the superficial fascia consists of a single layer. Below the umbilicus, the fascia divides into 2 layers: the Camper fascia (a superficial fatty layer) and the Scarpa fascia (a deep membranous layer). The superficial epigastric neurovascular bundle is located between these 2 layers. The abdominal subcutaneous fat, which is separated by the Scarpa fascia, is highly variable in thickness. The clinical relevance of this anatomy is appreciated when designing superficial inferior epigastric artery (SIEA) flaps. The SIEA flap has been used as a pedicled flap for hand reconstruction or as a free flap in breast reconstruction.
Deep fascia
The deep fascia is a thin, tough layer that surrounds and is adherent to the underlying abdominal muscles. Each abdominal muscle has an aponeurotic component that contributes to the deep fascia. The individual abdominal muscles are described below.
Subserous and peritoneal fascia
The subserous fascia is also known as extraperitoneal fascia and serves to bind the peritoneum to the deep fascia of the abdominal wall or to the outer lining of the gastrointestinal tract. It may receive different names depending on its location (eg, transversalis fascia when it is deep to that muscle, psoas fascia when it is next to that muscle, iliac fascia). The peritoneum is a thin (one-cell thick) membrane that lines the abdominal cavity. It is useful in reconstructive efforts because it provides a layer between the bowel and mesh. In addition, studies have demonstrated the utility of the thin, pliable, peritoneal-lined rectus flap in vaginal wall reconstructions.
Musculofascial layer
The abdominal wall includes 5 paired muscles (3 flat muscles, 2 vertical muscles). The 3 flat muscles are the external oblique, internal oblique, and transversus abdominis. The 3-layered structure, combined with extensive aponeuroses, works in a synkinetic fashion not only to protect the abdominal viscera but also to increase abdominal pressure, which facilitates defecation, micturition, and parturition. The 2 vertical muscles are the rectus abdominis and pyramidalis. Fusion of the fascial layers of these muscles forms 3 distinct fascial lines: the linea alba and 2 semilunar lines. The linea alba is formed by the fusion of both rectus sheaths at the midline, while the semilunar lines are formed by the union of the external oblique, internal oblique, and transversus abdominis aponeuroses at the lateral borders of the rectus abdominis muscles.
External oblique
The external oblique muscle is the largest and thickest of the flat abdominal wall muscles. It originates from the lower 8 ribs, interlocks with slips of latissimus dorsi and serratus anterior, and courses inferior-medially, attaching via its aponeurosis centrally at the linea alba. Inferiorly, the external oblique aponeurosis folds back on itself and forms the inguinal ligament between the ASIS and the pubic tubercle. Medial to the pubic tubercle, the external oblique aponeurosis is attached to the pubic crest. Traveling superior to the medial part of the inguinal ligament, an opening in the aponeurosis forms the superficial inguinal ring. The innervation to the external oblique is derived from the lower 6 thoracic anterior primary rami and the first and second lumbar anterior primary rami.
Internal oblique
The internal oblique muscle originates from the anterior portion of the iliac crest, lateral half to two-thirds of the inguinal ligament, and posterior aponeurosis of the transversus abdominis muscle. The internal oblique fibers run superior-anteriorly at right angles to the external oblique and insert on the cartilages of the lower 4 ribs. The anterior fibers become aponeurotic at around the ninth costal cartilage.
At the lateral border of the rectus abdominis muscle and above the arcuate line, the aponeurosis splits anteriorly and posteriorly to enclose the rectus muscle to help form the rectus sheaths. However, below the arcuate line, the internal oblique aponeurosis does not split, resulting in an absent posterior rectus sheath. The inferior aponeurotic fibers arch over the spermatic cord, pass through the inguinal canal and then descend posterior to the superficial ring to attach to the pubic crest. The most inferior medial tendinous fibers fuse with the aponeurotic fibers of the transversus abdominis muscle to form the conjoint tendon, which also inserts on the pubic crest.
Transversus abdominis
The transversus abdominis muscle is the innermost of the 3 flat abdominal muscles. The fibers of the transversus abdominis course predominantly in a horizontal orientation. It has 2 fleshy origins and 1 aponeurotic origin. The first fleshy origin is from the anterior three fourths of the iliac crest and lateral third of the inguinal ligament, while the second origin is from the inner surface of the lower 6 costal cartilages where they interdigitate with fibers of the diaphragm. Between the 2 fleshy origins is the aponeurotic origin from the transverse processes of the lumbar vertebrae. These fibers course medially to the lateral border of the rectus muscle. From about 6.6 cm inferior to the xiphoid process to the arcuate line, the insertion is aponeurotic and contributes to the formation of the posterior rectus sheath.
Rectus abdominis
The rectus abdominis muscles are paired, long, straplike muscles that are the principal vertical muscles of the anterior abdominal wall. The rectus abdominis is interrupted throughout its length by 3-4 tendinous inscriptions, all of which are adherent to the anterior rectus sheath and separated by the linea alba. These inscriptions can be visualized externally in a well-developed individual secondary to fasciocutaneous ligaments.
The medial tendon of the rectus abdominis originates from the pubic symphysis and the lateral tendon of the rectus abdominis originates from the pubic crest. It inserts to the anterior surfaces of the fifth, sixth, and seventh costal cartilages and xiphoid process. The lateral border of each rectus muscle and its sheath merge with the aponeurosis of the external oblique to form the linea semilunaris. The rectus abdominis muscle functions as a tensor of the abdominal wall and flexor of the vertebrae. Additionally, this muscle helps to stabilize the pelvis during walking, protects the abdominal viscera, and aids in forced expiration.
The rectus sheath is a strong, semifibrous compartment that houses the rectus muscles, the superior and inferior epigastric vessels, and the inferior 5 intercostal and subcostal nerves. It is formed by interlacing aponeurotic fibers from the 3 flat abdominal muscles. The anterior rectus sheath is the union of the external oblique aponeurosis and the anterior layer of the internal oblique. The posterior rectus sheath is composed of the posterior layer of the internal oblique aponeurosis, the transversus abdominis aponeurosis, and the transversalis fascia. Superior to the costal margin, the posterior rectus sheath is absent because the internal oblique muscle is attached to the costal margin and the transversus abdominis courses internal to the costal cartilages.
Pyramidalis
The pyramidalis is a small triangular muscle located anterior to the inferior aspect of the rectus abdominis; the pyramidalis is absent in about 20% of the population. The pyramidalis originates from the body of the pubis directly inferior to the insertion of the rectus abdominis and inserts into the linea alba inferior to the umbilicus to assist in stabilization of the lower midline.
Arcuate line
Above the arcuate line, the anterior rectus fascia exists anterior to the rectus muscle, and the posterior rectus fascia is posterior to the rectus muscle. Below the arcuate line, the 3 aponeuroses merge together to form exclusively the anterior rectus sheath, with little or no posterior sheath. The arcuate line is generally located 2 fingerbreadths from the umbilicus to midway between the umbilicus and pubis. However, some reports in the literature state that the arcuate line is closer to 75% of the distance between the pubic crest and the umbilicus or 1.8 cm superior to the ASIS.
Linea alba
The linea alba is the fusion of the anterior and posterior rectus fascia; it is located in the abdominal midline, between the rectus muscles, from the xiphoid to the pubis. The linea alba is a 3-dimensional composition of tendon fibers from abdominal wall muscles. Midline insertions of these fibers play a significant role in stabilizing the abdominal wall. The cranial aspect is attached to the xiphoid process, while, caudally, it inserts at the pubic symphysis.
Linea semilunaris
The linea semilunares can be seen as a pair of linear impressions in the skin that correspond with the most lateral edges of the rectus abdominis. These lines are visible in a person who is physically fit but obscured in a person who is obese. They are formed by the band of aponeuroses of the external oblique, the internal oblique, and the transversus abdominis muscles.
Vascular supply and innervation
The plane between the internal oblique muscle and transversus abdominis muscle contains the neurovascular structures that supply the abdominal muscles. The superior and inferior deep epigastric vessels enter the rectus muscle superiorly and inferiorly. Transperitoneal vessels enter the rectus in the periumbilical region. The abdominal wall receives its blood supply from direct cutaneous vessels and musculocutaneous perforating vessels. [8] The 2 subdivisions of perforators course medially and laterally. The lateral branch is usually the dominant branch and contains most of the perforator vessels. [9] The lateral fasciocutaneous perforators pierce the aponeuroses of the internal and external oblique muscles. They may pass through the linea alba and emerge on the lateral aspect of the rectus abdominis. [10, 11]
El-Mrakby et al performed microdissections to analyze the vascular anatomy of the anterior abdominal wall. They concluded that the musculocutaneous perforators are the main providers of blood supply to the anterior abdominal wall. [12] In addition, the vessels were further categorized into large (direct) or small (indirect) perforators. The indirect perforators generally have diameters less than 0.5 mm and terminate in the deep layer of the subcutaneous fat. [12] Conversely, the direct perforators have diameters greater than 0.5 mm and course into the subdermal plexus to supply the superficial subcutaneous fat and skin. [12] In addition, El-Mrakby et al described the area lateral and inferior to the umbilicus as the area with the richest concentration of perforator vessels. [13] This vascular network allows multiple flap designs that may incorporate one or several perforator vessels.
A study by Huger et al classified the vascular blood supply of the abdominal wall into 3 simple zones for abdominoplasty. [14]
Zone I is defined by the mid abdomen and is supplied primarily by the deep epigastric arcade. As the internal thoracic artery passes behind the costal cartilages to enter the abdominal wall, it gives rise to the superior epigastric artery. This vessel then enters the abdomen and travels underneath the surface of the posterior rectus sheath. The superior epigastric artery joins the deep inferior epigastric artery through a series of choke vessels within the rectus above the umbilicus.
Zone II is defined by the lower abdomen and is supplied by branches of the epigastric arcade and the external iliac artery. Blood supply superficial to the fascia is provided by the superficial epigastric and superficial pudendal arteries. Both of these arteries originate from the femoral artery. The deep iliac circumflex artery originates from the external iliac and runs deep to all abdominal muscles to provide blood supply to the area of the anterior iliac spine; it also pierces all 3 muscles of the lateral abdominal wall and provides a sizable musculocutaneous perforator.
Zone III comprises the flanks and lateral abdomen. Blood supply to this area comes from the intercostal, subcostal, and lumbar arteries. The intercostal vessels leave the rib cage and enter the abdominal wall between the transversus abdominis and internal oblique muscles, where they anastomose with the lateral branches of the superior epigastric artery and deep inferior epigastric artery.
Sensory innervation to the abdomen is derived from the roots of the nerves T7 to L4. These nerves travel in the plane between the internal oblique and transversus abdominis muscles. Motor innervation is provided by the intercostal, subcostal, iliohypogastric, and ilioinguinal nerves. These nerves must be preserved during abdominal wall reconstruction in order to maintain abdominal wall sensation and muscular function.
See Regions and Planes of the Abdomen for more information.
Contraindications
True contraindications to hernia repair are rare. A subset of minimally symptomatic or asymptomatic patients with very large hernias at very low risk for incarceration may benefit from observation rather than attempted repair. Additionally, in some patients, the cardiopulmonary stress or bleeding risk associated with major surgery may preclude operative management of their abdominal wall defect. Given the risk factors associated with the development of a ventral hernia, many of the potential candidates for repair have significant medical comorbidities. It is incumbent on the reconstructive surgeon to maintain an awareness of these comorbid conditions, as well as the alterations in physiology caused by the hernia defect or its repair.
Patients with conditions such as chronic obstructive pulmonary disease (COPD), heart disease, or liver failure must be preoperatively screened. Postoperatively, patients with COPD may be difficult or impossible to wean from the ventilator. Intra-abdominal operations involve large fluid shifts and may cause significant cardiovascular stress intraoperatively and postoperatively, with intravascular return of third space volume in the early postoperative period. In addition, patients with liver failure have high morbidity and mortality rates with operations that require general anesthesia and should not undergo elective abdominal wall reconstruction. The risk of such a major operation for patients with the above comorbid conditions must be defined, as these risks may outweigh the benefit of abdominal wall reconstruction.
Relative contraindications to elective abdominal wall reconstruction/ventral hernia repair include preexisting conditions that may increase the risk of recurrence (ie, smoking, mild COPD, obesity, diabetes, ascites, cancer, multiple hernia recurrences, a noncompliant patient). For more information on these conditions, visit the following Medscape Resource Centers: Smoking, COPD, and Diabetes.
-
This drawing illustrates the components separation technique. A longitudinal incision is made at the semilunar line, and the relative vascular plane is dissected between the external and internal oblique muscles. Incising the anterior rectus sheath allows for advancement of musculofascial components medially.
-
Forty-six-year-old patient with chest wall recurrence of primary visceral carcinoma requiring composite resection.
-
Resultant complex abdominal wall defect following resection. Note repair of the diaphragm to the revised costal margin and exposure of the liver.
-
Coverage of the open abdominal defect with a Parietex composite mesh (Covidien; Norwalk, CT).
-
Soft tissue closure of defect with right-sided pectoralis muscle flap, left myocutaneous latissimus flap, and "4-quadrant" fasciocutaneous advancement flaps.
-
Hernia grading system: assessment of risk for surgical site occurrences. Wound infection defined as being contained within the skin or subcutaneous tissue (superficial), or involving the muscle and/or fascia (deep).