Practice Essentials
Failure to thrive is an interruption in the normal pattern of growth, usually seen in younger children. While many anthropometric standards have been employed to define "growth," (body mass index [BMI], weight, or weight-for-height z-scores), serially comparing a child to appropriate standard growth curves is now commonly employed. (See the image below.)
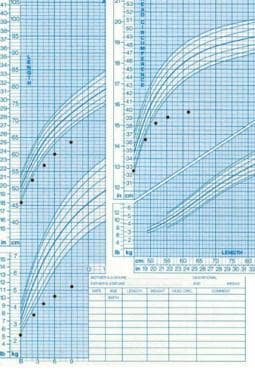 Failure of growth in weight, length, and head circumference starting at birth, suggesting an organic etiology that occurred in utero.
Failure of growth in weight, length, and head circumference starting at birth, suggesting an organic etiology that occurred in utero.
Often exacerbating the effects of chronic infections, failure to thrive is perhaps the greatest contributor to worldwide childhood morbidity and mortality. Stunting with intellectual compromise is seen in children who survive long periods of inadequate growth.
In the developed world, there is controversy regarding the long-term deficits seen in children who experience failure to thrive. However, the contribution that failure to thrive plays in contributing to the morbidity of pediatric pathologic states is being more appreciated.
Failure to thrive can be secondary to inadequate intake of calories, inefficient utilization of ingested calories (emesis, malabsorption), or an increased basal metabolism (usually seen in oncology, infections, cardiopulmonary deficiencies, chronic inflammatory states, and hyperthyroidism).
Comprehensive evaluation of children by multidisciplinary feeding teams has illustrated that frequently more than one entity is present in a single child with failure to thrive. One example would be the anorexia related to depression seen in children with chronic illnesses.
It has been long considered dogma that the overwhelming majority of children with growth impairment in the developing world have psychosocial issues that result in nonorganic failure to thrive. Multiple feeding teams have noted that among children with nonorganic failure to thrive, there is frequently a subtle organic problem such as dysphagia, gastroesophageal reflux, constipation, or food allergy/intolerance that has created pain and/or fear in this cohort. Recognition and therapy that addresses these issues have yielded improved outcomes.
Background
Failure to thrive (FTT) is both a descriptive term for various entities and a diagnosis. It is defined as a significant interruption in the expected rate of growth during early childhood. Because sequential measurements of growth are vital aspects of preventive pediatrics, FTT is a concern for all pediatric heath care providers. All standard pediatric textbooks have sections on this topic, [1] and numerous review articles have been written. [2, 3, 4, 5, 6] However, despite significant attention, the consequences of FTT on developmental outcomes in industrialized children is controversial, as discussed in the section on prognosis below. It is easier to appreciate that in certain children, FTT can be a prelude to significant physical and cognitive morbidity, including stunting, and mortality. This is especially relevant throughout the developing world, in rural and impoverished inner city children, and in those with multiple chronic illnesses. Two significant developments in the approach to the child with FTT have begun to dramatically influence the approach to these children.
Firstly, while it is accepted that all children with failure to thrive have inadequate or worsening growth over time, one area of controversy is determining which anthropometric criteria should be utilized to define this term. [7] The most common definitions are weight less than the third to fifth percentile for age on more than one occasion or weight measurements that fall 2 major percentile lines using the standard growth charts of the National Center for Health Statistics (NCHS).
Some authors have included height measurements as part of the definition; however, height measurements more precisely describe short stature. If weight parameters are significantly compromised, height can also be secondarily affected in individuals with FTT. A European study examined a large cohort of children using various terms associated with pediatric growth compromise and documented a wide variance in the prevalence of this condition. [8] Although serial measurements of head circumference are important in the evaluation of infants and toddlers, isolated failure of the head to grow should not suggest the typical failure to thrive differential.
The American Society for Parenteral and Enteral Nutrition assembled a group of experts that published a comprehensive report on pediatric malnutrition based on a comprehensive analysis of literature published through 2011. [9] They organized their review, discussion, recommendations, and definition around five key domains. The authors developed a novel definition of pediatric malnutrition that clearly overlaps with the term failure to thrive as employed in this article and by multiple other authors. Their proposed definition is an “imbalance between nutrient requirements and intake, resulting in cumulative deficits of energy, protein, or micronutrients that may negatively affect growth, development, and other relevant outcomes."
To address the controversy of determining which anthropometric criteria should be utilized to define FTT, the group has recommended that z-scores be used to express individual anthropometric variables in relation to the population reference standard. The authors agree that this is the best approach to track serial measurements in a child being evaluated for FTT.
The second new development in the approach to a child with FTT is that especially since 2011, there have been a number of publications from multidisciplinary feeding groups from around the United States that have stressed the significant integration between physical issues and psychosocial problems that yield FTT (see pathophysiology section below).
The working group characterized failure to thrive as “a term used to describe children who are not growing as expected.” They note that more than 90% of cases in most studies do not have an underlying medical cause, and virtually all are identified by a careful history and physical examination. [10] The report also cites a paper describing an inner city academic center that only evaluated 75 children in their specialized clinics over a 40 month period. These authors and others have characterized virtually all of their referrals as having psychosocial etiologies for their FTT and recommended a reappraisal of how to provide resources for these children. [11]
Another study from a multidisciplinary feeding program at a US medical school also reports that 90% of their referrals had nonorganic failure to thrive. While they described a very short follow-up, they also report that children who adhered to their basic instructions were able to quickly gain significantly more weight than those were “nonadherent.” However, the 25% of the cohort who were unable to follow directions, may also include children who had unidentified issues. [12]
With the routine inclusion of comprehensive speech and feeding evaluations as part of the FTT work-up, psychosocial compromise is now recognized as most likely to yield failure to thrive in children with subtle swallowing dysfunction, or other primary organic conditions, especially those associated with visceral pain during feeds. [13] As this fundamental paradigm is reconsidered, [14, 15] the practicing provider evaluating a deprived child with failure to thrive must make every effort to illicit and correct discomfort (such as feeding refusal, grimacing, bloating, premature satiety) or dysphagia (such as coughing, choking, gagging, or extended meal times). Thus, a routine part of the evaluation of children with failure to thrive is identifying any pathophysiologic conditions that may contributing factors.
To consolidate all of these nuances, some authors have gone from categorizing children as either one extreme or the other to describing a spectrum extending from pure nonorganic failure to thrive to pure organic failure to thrive, with individual children lying closer to one or the other. [16] In this view, medical, developmental, neurological, and environmental issues are often found in a single child. [17]
Normal growth and growth charts of term and premature infants, as well as the etiology, evaluation, management, and outcome of failure to thrive are discussed in this article. For information on energy malnutrition, see the article Marasmus.
Pathophysiology
Organic FTT is secondary to inadequate intake, increased losses, compromised utilization of ingested calories, excessive metabolic demands, or combinations of these factors. Nonorganic failure to thrive is defined as a consequence of inadequate energy intake. However, the complexity of inadequate intake has now been studied in more detail. While classically viewed as a behavioral (ie, nonorganic) problem, it is now appreciated that identifiable and treatable pathologies can be antecedents of this behavior and must be eradicated to reverse this situation. [18] Conversely, the increased prevalence of FTT in children with chronic medical conditions has been emphasized by the recent pediatric malnutrition working group, which recommended that malnutrition be characterized explicitly with reference to the specific pathologic state. [9] The following examples illustrate the spectrum of potential interactions between organic and nonorganic features in children with FTT. [19, 20, 21, 22, 23, 24, 25]
Factors contributing to childhood feeding difficulties were described in 340 out of 349 participants identified by an interdisciplinary feeding team. These included developmental disabilities, gastrointestinal problems, cardiopulmonary problems, neurological problems, renal disease, and anatomical anomalies. The most prevalent condition was gastroesophageal reflux. [19]
In a review of 38 intervention studies of 218 children with food refusal, 212 had some form of a medical issue. The majority, 116 children (55%), had gastrointestinal disorders (eg, gastroesophageal reflux) and 131 (62%) had a non-gastrointestinal medical disorder (eg, bronchopulmonary dysplasia, seizure disorder). In this study gastroesophageal reflux was the most common medical diagnosis, while other diagnoses such as cardiopulmonary conditions (33%), neurological conditions (25%), food allergies (15%), anatomical anomalies (14%), and delayed gastric emptying (6%) were also reported. [20]
Another study examining sensory processing skills in a childhood feeding disorders group of toddlers with nonorganic failure to thrive concluded that sensory processing problems were more commonly observed in toddlers with feeding problems and growth deficiency. [21]
Out of 143 subjects in a feeding clinic in a tertiary hospital, 65.5% (55/84) of children with a medical condition also had one or more feeding behavior disorders. This study by Jung et al also found no underlying medical disorder to explain the feeding disorders in 59 (41.3%) of the 143 subjects. Many of these children had feeding behavior disorders such as sensory food aversion and infantile anorexia with FTT. [22]
Dysphagia or odynophagia—caused by the inflammation of the esophagus (drug-induced, allergic, eosinophilic, or induced by gastroesophageal reflux disease), motor dysfunction, or structural anomaly of the upper gastrointestinal tract—is well recognized as a cause of food refusal, as swallowing food becomes a triggering factor of pain. [23, 24]
In a study characterizing the feeding difficulty of 700 children referred for assessment of severe feeding difficulty, close to 50% of the children had a combined medical and oral condition underlying their feeding difficulties. More than half of the children were examined for gastrointestinal conditions, particularly gastroesophageal reflux disease. The results indicate that oral sensory-based feeding problems are related to past medical intervention. [25]
Normal growth in term infants
The average birth weight for a term infant is 3.3 kg. Weight drops as much as 10% in the first few days of life, secondary to loss of excess fluid. By 10-14 days of life, birth weight should be regained. Breastfed infants who are fed smaller volumes of colostrum for the first few days regain birth weight a little later than bottle-fed infants.
On average, infants gain 1 kg/mo for the first 3 months, 0.5 kg/mo from age 3-6 months, 0.33 kg/mo from age 6-9 months, and 0.25 kg/mo from age 9-12 months. Expressed in another perspective, full term infants double their birth weight by 4-6 months of age and triple their weight by 12 months of age. A third approach to use is that term infants gain almost 30 g (1 oz) per day for 3 months and then almost 15 g (0.5 oz) per day for the next 6 months. From 9 months of age until the child is a toddler, the average weight gain is roughly 0.25 kg/mo (or 0.5 lb/mo). Afterwards, the weight gain is about 2 kg/y through early school age.
Caloric requirements to assure adequate intake in a normal infant is 100-110 kcal/kg/d for the first 6 months and decreases slightly to 100 kcal/kg/d for the second half of the first year. Beyond 10 kg, 50 kcal/kg/day is required until 20 kg and beyond 20 kg, 20 kcal/kg/d are necessary.
Term infants grow 25 cm in length during the first year, 12.5 cm in the second year, and then slow down to approximately 5-6 cm between 4 years of age and the onset of puberty, at which time, growth can increase up to 12 cm per year.
The average head circumference is 35 cm at birth and rapidly increases to 47 cm by age 1 year. The rate of growth then slows, reaching an average of 55 cm by 6 years of age.
Also, the upper-to-lower body segment ratio changes with growth. Normally, the ratio at birth is 1.7, the ratio at 3 years of age is 1.3, and the ratio by 7 years of age becomes 1.0 with the upper body segment and lower body segment being about equal. The lower body segment is measured from the symphysis pubis to the floor.
Normal growth in premature infants
When plotting growth charts for premature babies, a "corrected age" should be used. This corrected age can be calculated by subtracting the number of weeks of prematurity from the postnatal age. Special growth charts based on gestational age rather than chronological age have been developed for infants, beginning at 26 weeks' gestational age. However, because these charts represent a compilation of a relatively small number of infants, they may not be completely reliable. Whichever technique is used for premature babies (eg, adjustment of age, using specific premature growth charts), consistency of methodology is essential. Once a method for plotting growth is chosen, that technique should be followed each time plotting occurs. Prior to 40 weeks' gestation, some infants may require as much as 120 kcal/kg/d to ensure adequate weight gain.
Catch-up growth is attained at approximately 18 months of age for head circumference, 24 months of age for weight, 40 months of age for height. Subsequently, normal growth charts can be used. In some premature babies with very low birth-weight, catch-up growth does not occur until early school age.
Growth charts
Growth charts were developed by the NCHS based on data collected through the Third National Health and Nutrition Examination Survey. They have been used since 1977 and are available for males and females 0-36 months of age and 2-18 years of age. The growth charts for boys and girls 0-36 months of age include weight and height for age and head circumference; growth charts for both age groups include weight for stature.
These charts have been revised and are available from the Centers for Disease Control and Prevention (2000 CDC Growth Charts: United States). [26] The new charts are applicable to infants, children, and adolescents from birth to age 20 years and have 7 percentile curves (5th, 10th, 25th, 50th, 75th, 90th, 95th). Charts are available for use in subspecialty patients (eg, endocrine, gastroenterology), with additional third and 97th percentile curves. Body mass index (BMI) charts, which are available for individuals aged 2-20 years, have replaced the weight-for-stature charts. BMI is calculated by dividing weight in kilograms by height in meters squared.
Accurate measurements are essential to the interpretation of growth charts. Scales need to be regularly calibrated; length should be carefully measured, and head circumference should be measured using standardized techniques.
The World Health Organization (WHO) has recommended the use of z-scores in expressing anthropometric measurements. Z-scores allow more precision in describing anthropometric status than does the customary placement “near” or “below” a certain percentile curve. [9]
Alternate growth charts are available for children who are breastfed and for children with multiple genetic conditions including Down syndrome, [27] Turner syndrome, [28] achondroplasia, [29] meningomyelocele, low birth weight, and very low birth weight. No matter which growth chart is used, the most valuable information is obtained by careful measuring and plotting on the same chart over time. Infants and children should remain within 1-2 percentile curves over time.
Other anthropometric variables for assessing nutritional status
In acutely ill children, height and weight measurements may be challenging to accurately record due to difficulty in transporting critically ill children, or to fluid shifts that affect the measurement. Mid–upper arm circumference (MUAC) has been suggested as a proxy for weight and HC as a proxy for height. In patients with fluid shifts and edema, MUAC may be a better indicator than weight-for-height for classification of acute malnutrition. MAMC and triceps skinfold are other measurements that are useful in children where height and weight measurements are not easily acquirable. Mid-arm muscle circumference (MAMC) may be calculated from MUAC and triceps skin fold (TSF) using the formula MAMC = MUAC – (TSF × 0.314). [9]
Etiology
Failure to thrive can be organized into nonorganic failure to thrive, organic failure to thrive, and a combination of nonorganic and organic failure to thrive. The relative incidence of each category completely depends on the population that the study examines. A study from a pediatric endocrinology clinic in a US university hospital found that half of the patients had a purely nutritional deficiency and another quarter had short stature. [30] As indicated above, many of the children from older articles who were considered to have nonorganic failure to thrive actually had subtle organic problems that contributed to their poor growth.
While the authors believe that most children have contributions from both etiologies, guidelines are being composed to assist clinicians with unclear circumstances. A retrospective review compiled by a national children's hospital in Korea concluded that in their population, children with organic failure to thrive had significantly lower gestational ages, birth weights, and weight percentiles at presentation than those with nonorganic failure to thrive. In addition, the children with organic failure to thrive were more likely to have severe weight decline. [31]
Nonorganic failure to thrive
Nonorganic failure to thrive, the most written about form of failure to thrive results from adverse environmental and psychosocial factors. [32] The onset is almost always prior to age 5 years. It is often associated with abnormal interactions between the caregiver and the infant or child. At times, it can be part of a more global pattern of child abuse. The result is an inadequate provision of food and/or inadequate intake of food. It is most common in the setting of poverty. When considering almost all of the individual entities associated with nonorganic failure to thrive, an organic process can frequently be identified as an accomplice.
Prenatal causes of nonorganic failure to thrive include the following:
-
Some mothers who are malnourished during pregnancy can have babies who are malnourished and small. This is more common in teen pregnancies, in lower socioeconomic locations, and with multiple gestations.
-
Maternal eating disorders (eg, anorexia, bulimia) can affect the growth of fetuses as well. Whether the failure to thrive in these infants is related to hormonal factors in addition to nutrient deprivation is open to debate.
Postnatal causes of nonorganic failure to thrive include the following:
-
Traditionally, nonorganic postnatal causes of failure to thrive were thought to be due to maternal rejection or neglect. Skuse et al suggested that clinicians inquire about more than just the nutrition offered to children. [33] He found behavior at meals and psychosocial issues to be important variables that affected whether children obtained sufficient energy.
-
Poor parenting and family dysfunction can negatively affect a child's energy intake. Families characterized by less adaptive relationships, higher levels of family conflict, maternal drug abuse, maternal depression, lack of maternal education, and less emotional support for the mother have an increased rate of children with failure to thrive. The term psychosocial deprivation was created for these types of situations.
-
Other classical nonorganic reasons for failure to thrive in younger children include a failure to signal hunger, a poor suck, difficulty in weaning, or a refusal to eat. An organic basis that plays a large role in these behaviors is now noted. An appreciation of the role of sensory integration dysfunction in a subset of children with these suck and signaling problems highlights the difficulty in separating organic from nonorganic FTT. [21]
-
Rarely in infants and toddlers, but more common in older children, eating disorders (eg, anorexia, bulimia) may lead to severe growth disturbances. Although infants and toddlers do not have the classical disturbed body image that characterizes the adolescents with eating disorders, all are involved in a struggle over control with food as the medium.
-
A retrospective review from an Asian children's hospital found the most common history for children with nonorganic failure to thrive was a preceding intercurrent viral illness followed by adoption of ill-advised compensatory feeding measures by the care provider. [31]
-
In cohorts where the prevalence of FTT is high, efforts should be made to understand patterns of complementary feeding and to realize that complementary food choices are culturally dependent. [34]
-
Although the typical groups with nonorganic failure to thrive are infants and toddlers, the younger the child is, the more likely they are to have some organic pathology that contributes to the aberrant feeding behavior. Children who are slow or fussy feeders often result in parental frustration and a lack of persistence. Underlying mild dysphagia may be part of the cause of this behavior. Similarly, feeding aversion may start as a consequence of pain related to gastroesophageal reflux, enteropathy or fear of micro-aspiration in an infant. Subsequently, the fear may persist even after effective medical therapy and become more consequential than the original organic problem. [35]
-
Mild dysphagia is present, the infant may be a slow or fussy feeder, which can lead to parental frustration and their lack of persistence. Conversely, pain related to gastroesophageal reflux or enteropathy or fear of aspiration may lead to feeding aversion by the infant that becomes a more significant problem than the organic one.
Nonorganic causes of failure to thrive usually include combinations of the following:
-
Poverty
-
Dysfunctional family interactions (especially maternal depression or drug use)
-
Difficult parent-child interactions
-
Lack of parental support (eg, no friends, no extended family)
-
Lack of preparation for parenting
-
Family dysfunction (eg, divorce, spouse abuse, chaotic family style)
-
Difficult child (prior to this characterization, the provider should seek out explanations as to why, including subtle dysphagia)
-
Child neglect
-
Emotional deprivation syndrome
-
Inadequate understanding of age appropriate acquisition of mealtime feeding skills by the care provider (ie. failing to introduce chewable solid foods and finger feeding at critical periods).
-
Responding inappropriately in mealtime situations to the child's negative behaviors like tantrums, expelling or throwing food, and inadvertently ending the meal which allows the child to terminate eating and reinforces the behavior.
-
Feeding disorders (eg, anorexia, bulimia)
Organic failure to thrive
Prenatal onset of organic failure to thrive involves the following:
-
Prenatal causes of failure to thrive are often associated with complications of prematurity. Premature babies have an increased incidence of many medical conditions, including renal disease, heart disease, lung disease, and CNS disorders. All of these disorders can lead to intrauterine failure to thrive.
-
Most premature infants catch-up to the growth of term babies by the time they are aged 2-4 years. Intrauterine growth retardation (IUGR) is diagnosed when an infant is born below the expected weight (usually < 3%) for their gender and gestational age. Some premature (as well as full term) babies, particularly those with concomitant IUGR, have failure to thrive.
-
Whether premature babies are small because of prematurity or whether they have permanent failure to thrive is sometimes difficult to determine. If infants double their birth weight by age 4-6 months and triple their birth weight by 1 year, then full catch-up growth can be anticipated.
-
Other causes of the prenatal onset of failure to thrive include exposure to toxins, environmental influences, maternal factors, intrauterine infection, and placental or chromosomal abnormalities.
-
The most important prenatal exposures compromising growth are tobacco, which is known to produce placental insufficiency, and alcohol ingestion. Prenatal ingestion of drugs of abuse (eg, cocaine, amphetamines) can also play a role in the prenatal onset of failure to thrive. Because these drugs are often taken together, separating the effects of each drug may be difficult. Also, maternal exposure to certain medications (eg, hydantoin, phenobarbital) can lead to in utero failure to thrive.
-
Certain maternal illness (eg, hypertension, preeclampsia, heart disease, anemia, advanced diabetes mellitus) can lead to uteroplacental insufficiency and can result in smaller babies.
Although the differential diagnosis of postnatal organic failure to thrive is vast, dividing the etiology is useful. The etiology can be divided into the following 3 general areas: inadequate energy intake, compromised use (usually vomiting or malabsorption and/or excessive losses), and excessive metabolic demands. An astute mother recognizes the category to which her baby belongs. The astute physician recognizes patterns that encompass more than one of these categories.
Causes of inadequate energy intake include the following:
-
These causes can result from mechanical problems (eg, neuromuscular abnormalities, craniofacial abnormalities), lack of appetite, breathing difficulties, significant developmental delay, and primary GI disease or dysfunction. Medical therapy itself can sometimes significantly affect intake.
-
Mechanical problems can cause a poor suck or defective swallowing. Hypotonia (eg, Wernig-Hoffman syndrome, Prader-Willi syndrome), neuromuscular or CNS system disease, and CP (most commonly) lead to incoordination of feeding. Structural defects related to craniofacial abnormalities (eg, severe micrognathia, cleft palate, cleft lip) make coordinating an oral bolus difficult. Children with developmental disabilities are often malnourished.
A recently published analysis of growth data from a multicenter US neonatal research network concluded that children with hypoxic ischemic encephalopathy and moderate-to-severe CP had increased prevalence of weight, height, and head circumference greater than 10% at age 6-7 years compared with children without CP. [36]
However, a systematic approach can identify specific problems, including the need for supplemental gastrostomy tube feeds, and, once corrected, a considerable benefit is realized. [37]
-
Children who have chronic illnesses are often too sick or too apathetic to maintain good oral intake. A lack of appetite is seen in renal failure, malignancy, tuberculosis, and HIV infection, which are associated with increased circulating levels of cachectin (also known as tumor necrosis factor [TNF]).
-
Chronic cardiopulmonary compromise can make feeding exhausting and can attenuate caloric intake. Examples include congenital heart disease that leads to congestive heart failure (CHF) or chronic lung disease (eg, bronchopulmonary dysplasia, cystic fibrosis).
-
Conditions that cause abdominal pain with eating (eg, gastroesophageal reflux, celiac disease, inflammatory bowel disease, other enteropathies), and those that include impaired peristalsis (eg, achalasia, gastroparesis, pseudoobstruction) all decrease ingestion.
-
Children with developmental disabilities often have failure to thrive based on multiple physical and psychosocial contributions.
-
The discovery of new hormones that appear to play a role in the control of appetite has led to investigations on their potential role in failure to thrive. However, to date, no definitive conclusions can be drawn. Ghrelin is an orexigenic (appetite-stimulating) hormone that enhances intake of calories, thus it would be anticipated to induce a positive energy balance.
An investigation that examined blood levels of ghrelin in children with failure to thrive revealed higher circulating concentrations but paradoxically lower appetite scores. [38] .
Another study that measured serum levels of ghrelin and obestatin (an anorexigenic, appetite-decreasing hormone) also found no differences when comparing children with failure to thrive to normal-weight controls. [39]
A third study followed ghrelin and leptin (another recently discovered anorexic peptide) levels in a single child with failure to thrive related to the diencephalic syndrome and found that leptin levels fell when tumor size decreased and weight increased. [40] Although the authors correctly concluded that this was unexpected because leptin (which is mainly secreted by adipocytes, fat cells) is expected to increase, an alternative hypothesis could be that tumor removal may have somehow increased gastric secretion of leptin, potentially leading to weight gain.
Inadequate use of ingested energy includes the following:
-
This can cause failure to thrive even when oral intake is adequate. This is usually secondary to emesis, or malabsorption, which is sometimes secondary to compromised digestion.
-
Children with metabolic diseases, drug toxicities, gastroesophageal reflux, and eosinophilic, viral, or traumatic esophagitis may experience considerable vomiting and may be unable to salvage adequate quantities of their ingested caloric intake.
-
Malabsorption may be secondary to compromised villous surface area, such as in celiac disease (gluten enteropathy), tropical sprue, cow's milk allergy and (less commonly) soy protein allergy, postviral enteropathy, chronic giardiasis and other chronic parasites, immunoglobin A (IgA) deficiency, radiation enteritis, Crohn disease, severe iron or zinc deficiency, acrodermatitis enteropathica, small bowel lymphoma, bacterial overgrowth, Zollinger-Ellison syndrome, Whipple disease, and abetalipoproteinemia.
-
An entity that has been receiving an increased amount of consideration that can result in poor weight gain if not recognized is food protein–induced enterocolitis syndrome (FPIES). [41] This non–IgE-mediated hypersensitivity is most often triggered by cow milk or soy protein, but it can be a reaction to rice, oat, or other dietary antigens. Children present with repetitive emesis, often with diarrhea, which can cause dehydration and be mistaken for acute viral gastroenteritis. A delay in diagnosis can result in multiple episodes that may lead to failure to thrive.
-
Anatomic defects of the small intestine (eg, short gut syndrome, blind loop syndrome, bacterial overgrowth, Hirschsprung disease) are frequently associated with failure to thrive.
-
Digestion must precede absorption and any diseases that compromise this process can lead to wasting of energy and failure to thrive. Examples include pancreatic insufficiency including cystic fibrosis and (less commonly) Shwachman-Diamond syndrome and chronic cholestasis (seen primarily in children with hepatobiliary disease, especially biliary atresia) and cirrhosis secondary to metabolic disease and intrauterine infection
-
Excessive losses of protein from the gut can disrupt growth and is discussed in the article Protein-Losing Enteropathy.
Illnesses that increase metabolic demands include the following:
-
Cancer, HIV, inflammatory bowel disease, certain collagen vascular diseases, and cardiopulmonary deficits that lead to tachypnea and hyperthyroidism can increase the amount of basal energy expenditure and thus increase the amount necessary to achieve normal growth.
-
Many illnesses simultaneously have 2-3 features that cause failure to thrive. Children with CHF or bronchopulmonary dysplasia have both decreased intake of nutrients and increased metabolic demands. In addition, right-sided heart failure leads to digestive tract edema that compromises digestion and absorption. Children with cystic fibrosis often have tachypnea, frequent intercurrent illnesses that rob them of their appetite, and fat malabsorption. These children may also suffer from depression, which introduces another important contributor to failure to thrive.
An important part of the evaluation of all children is observation of the infant while feeding. This elucidates maternal-infant interactions, the infant's ability to suck and swallow, and the general health, development and fatigability of the child.
Genetic short stature and constitutional delay of growth are 2 conditions associated with decreased growth that must be distinguished from failure to thrive. From birth to about age 2 years, a baby's weight changes to follow the genetic predisposition of the parents' height and weight. During this time of transition, children with genetic short stature may cross percentiles downward and still be considered normal. However, most children in this category find their true growth curve by age 3 years. Although children with genetic short stature are often below the third percentile on the growth chart, they have normal weight-to-height ratios and bone ages equal to their chronological ages.
The other condition associated with short stature that must be distinguished from failure to thrive is constitutional growth delay, another variation of normal growth. Children with short stature resulting from constitutional delay often have a family history of delayed growth and puberty. They have a deceleration of growth in the first 2 years that can be confused with failure to thrive, but then grow parallel to but below the third percentile. Puberty is delayed, but ultimate height may be normal. A distinguishing point from genetic short stature is that bone age is delayed.
Table 1. Summary of Organic Causes of Failure to Thrive (Open Table in a new window)
Prenatal causes |
|
Postnatal causes |
Inadequate intake
Poor absorption and/or use of nutrients
Increased metabolic demand
|
Combined organic and nonorganic failure to thrive
Failure to thrive is now becoming more commonly recognized as the result of both organic and nonorganic reasons. [14, 15] In a recent multicenter study nearly one-half of children hospitalized for FTT had a complex chronic condition. However, the authors also realized that children with prematurity-related conditions and low median household income represent unique populations at higher risk for FTT readmissions. [42] Among the many infants with psychosocial deprivation, those with growth compromise often exhibit subtle evidence of dysphagia.
Conversely, some children with chronic illnesses who have failure to thrive may have additional psychosocial issues that compromise adequate treatment for their primary organic disease. These would include poverty, lack of education, concurrent family emergencies or natural disasters, and dysfunctional or ill caretakers, who may have psychological or behavioral co-morbidities themselves. Illnesses in children, particularly chronic illnesses, may create insurmountable psychological, emotional, and financial burdens for certain families. Stresses from coping with chronic illnesses may lead to parental dysfunction, such as depression, alcohol or drug abuse, divorce, or chaotic home environments. Parental dysfunction and the resultant negative atmosphere in which children are reared affect their food intake. Among the many infants with psychosocial deprivation, those with growth compromise often exhibit subtle evidence of dysphagia.
Children may also undergo personality changes when they have chronic diseases. Medications (eg, steroids) are well known to cause behavioral changes, but the mere presence of a chronic illness can also result in resistance or noncompliance in many aspects of a child's life, including consumption of proper energy intake. This is most common when the chronic illness includes the GI tract (eg, Crohn disease) or is especially debilitating (eg, HIV, difficult to treat neoplasia). As psychologists identify a greater proportion of children with chronic diseases who have depression as a comorbidity, this possible cause of failure to thrive should not be overlooked.
Special concerns and medicolegal issues
A recently published systematic review of failure to thrive in affluent societies reveals expected conclusions in this population. [43] Since psychosocial deprivation is very rarely encountered in this cohort, the incidence of failure to thrive is less than anticipated in an unselected group of children. In addition, most measurements that fall below the defined cutoff represent transient events leading to temporary weight loss rather than significant chronic problems. The authors correctly recommend that children from this demographic should only be evaluated for failure to thrive when they meet the criteria over an extended period, rather than simply making this diagnosis based on a single set of measurements.
Failure to identify a child with a potentially treatable cause of organic failure to thrive that leads to permanent sequelae secondary to a delay in diagnosis is a pitfall. Conversely, failure to refer a child who requires protective services while being evaluated for nonorganic failure to thrive who sustains an inflicted complication is yet another potential pitfall.
Perhaps the greatest challenge for healthcare providers evaluating children with failure to thrive is to identify if the family seeking medical input is thinking of the best interest of the child. Medical child abuse (MCA), a form of Munchausen syndrome by proxy, is defined as a caretaker seeking unnecessary and harmful or potentially harmful medical care and/or procedures. An article published in Pediatrics provides the most cogent guidelines to date, and physicians should familiarize themselves with the risk stratification tool developed by this group. [44]
The review was based on a retrospective chart review comparing 17 cases of MCA to 68 controls from the same location and the same years with failure to thrive but without MCA. Features that distinguished MCA patients were involvement of multiple subspecialists in children without congenital anomalies, parents seeking input from multiple institutions, parents refusing services from a multidisciplinary feeding team or not allowing social workers into their homes, siblings with similar complaints, and children with multiple allergies restricting their caloric intake and leading to multiple formula changes.
The MCA cohort were also subjected to significantly more invasive procedures (1) to make a diagnosis (including upper endoscopy and muscle biopsy) and (2) to provide supplemental calories (nasogastric tube feedings, gastrostomy tube placement, Nissen fundoplication, and placement of a central line for parenteral nutrition).
Their model was able to predict MCA with a sensitivity of 100% and a specificity of 96%. However, this application was based on a small number of MCA cases, not all of whom were definitively identified as having this problem. In addition, it was derived from a specialized referral unit and may not be universally applicable.
The authors do agree with the conclusions from this review that the presence of inconsistencies over time and the pattern of increasingly more invasive investigations to identify a cause of the failure to thrive, or the necessity of increasingly more invasive procedures to provide adequate calories is alarming. Despite the trend to minimize hospitalization in general, including as part of the failure to thrive algorithm, there are certain circumstances in which the child must be observed to determine the precise nature of the problem. These become more compelling when the experienced clinician is faced with clinical events that stray further and further from what would be expected.
Epidemiology
United States data
In reports from 1980-1989, failure to thrive accounted for 1-5% of tertiary hospital admissions for infants younger than 1 year. As many as 10% of children in primary care settings and 5% of US hospitalized children have been reported to show signs of failure to thrive. [30, 10] The incidence is highest in children with prematurity and with other medical conditions. The proportion of nonorganic failure to thrive among all infants with failure to thrive is much higher in the United States and other industrialized countries than in the developing nation.
International data
In underdeveloped countries, malnutrition manifesting as failure to thrive is more common. Worldwide, underweight prevalence was projected to decline from 26.5% in 1990 to 17.6% in 2015, a change of –34% (95% confidence interval [CI], –43% to –23%). In developed countries, the prevalence was estimated to decrease from 1.6% to 0.9%, a change of –41% (95% CI, –92% to 343%). In developing regions, the prevalence was forecasted to decline from 30.2% to 19.3%, a change of –36% (95% CI, –45% to –26%). In Africa, the prevalence of underweight was forecasted to increase from 24.0% to 26.8%, a change of 12% (95% CI, 8%-16%). In Asia, the prevalence was estimated to decrease from 35.1% to 18.5%, a change of –47% (95% CI, –58% to –34%). Worldwide, the number of underweight children was projected to decline from 163.8 million in 1990 to 113.4 million in 2015, a change of –31% (95% CI, –40% to –20%). Numbers are projected to decrease in all subregions except the subregions of sub-Saharan, Eastern, Middle, and Western Africa, which are expected to experience substantial increases in the number of underweight children. Thus, neither the world as a whole, nor the developing regions, achieved the Millennium Development Goals of decreasing the prevalence by 50% from 1990 to 2015. This is largely due to the deteriorating situation in Africa where all subregions, except Northern Africa, are expected to fail to meet the goal. [45]
Race-, sex-, and age-related demographics
Failure to thrive can occur in all socioeconomic strata, although it is more frequent in families living in poverty. Studies indicate increased incidence in children receiving Medicaid, children living in rural areas, and children who are homeless.
Nonorganic failure to thrive is reported more commonly in females than in males. In certain areas of the world where there is nutrient shortage, distribution is sometimes gender based, yielding greater prevalence in females.
The term is mainly reserved for growth compromise in young children.
Prognosis
Multiple studies have investigated whether failure to thrive is associated with long-term cognitive deficits. [46, 47, 48] Two published meta-analyses looking at cognitive outcomes of published children with failure to thrive in developed countries found small differences consisting of 3-4 intelligence quotient (IQ) points. [46, 48] Interestingly, one group concluded that this disparity was not enough to warrant an aggressive approach to identification and treatment of this entity. [46] The other authors suggested substantial population-based cognitive deficiencies could be attributed to failure to thrive. [48]
Another longitudinal population study of a large cohort found the same degree of IQ score difference when they examined a cohort with infantile failure to thrive. [49] A separate study that further divided nonorganic failure to thrive into those who had or had not experienced neglect defined a particularly vulnerable cohort; failure to account for this additional variable may explain some differences. [50]
A new area of research has been exploring whether aggressive refeeding or early malnutrition itself could impact future health parameters independent of simply changes in BMI and growth. The Barker or Fetal Origins Hypothesis is based on data accumulated over the last 20 years that has linked low birth weight to a subsequent increased risk for cardiovascular disease and type 2 diabetes. This theory states that in utero nutrient restriction results in epigenetic modifications that reprogram intermediary metabolism, glucose regulation, and blood pressure regulation. These genetic changes persist into adult life and yield increased susceptibility to disease.
A few epidemiologic studies have hypothesized that this principle could be extended to include malnutrition occurring in early postnatal life. A comprehensive review summarizes the published literature in this field and concludes that children with early-onset enteric infections, malnutrition, and stunting appear to be at increased risk to ultimately develop the metabolic syndrome. [51] A small observational study describes a cohort of young children with severe failure to thrive who received aggressive nutritional rehabilitation and ultimately developed obesity. [52] Whether this was a consequence of the primary deficit or the therapy is not addressed.
Potential long-term psychosocial consequences of stunting secondary to growth failure in early childhood in the developing world have been highlighted by a longitudinal study following a rural Guatemalan cohort. [53] Affected individuals went on to have profound consequences in adulthood related to economic status, marriage, and fertility. They scored worse on tests of reading and intelligence and had lower cognitive skills. Men had decreased likelihood of entry into higher-salaried positions. Individuals who were stunted often entered into relationships with poorer partners and were more likely to live in poorer households as adults. Women with stunting had their first child at a younger age and had more pregnancies and more children. While provocative, the potential for other economic, educational, or sociologic factors being the primary explanation for these outcomes still needs to be considered.
Although the goal of all pediatricians caring for children with organic failure to thrive is to incorporate measures into their management that are designed to preserve adequate growth, this may prove to be difficult. A greater appreciation for the significant prevalence of failure to thrive in children with multiple chronic illnesses including cerebral palsy (CP), congenital heart disease, cystic fibrosis, cirrhosis, HIV, inflammatory bowel disease, malignancy, and genetic diseases has been noted as well as the contribution of FTT to the ultimate outcomes in these patients.
Morbidity/mortality
The global trend in chronic failure to thrive (referred to as stunting) prevalence and numbers affected is decreasing. Between 2000 and 2013 stunting prevalence declined from 33% to 25% and numbers declined from 199 million to 161 million. In 2013, about half of all stunted children lived in Asia and over one third in Africa. Globally, 51 million children under-five years of age were acutely malnourished (wasted) and 17 million were severely wasted. Globally, wasting prevalence in 2013 was estimated at almost 8% and nearly a third of that was for severe wasting, totaling 3%. Approximately two thirds of all wasted children lived in Asia and almost one third in Africa, with similar proportions for severely wasted children. [54]
Ultimate physical growth and cognitive development may be decreased in children with long standing failure to thrive, especially with an early onset. However, efforts to analyze the published data have not yielded unequivocal confirmation in children in the developing world. [46] Earlier publications have described more cognitive deficits in nonorganic than organic failure to thrive. [55]
In developing countries, malnutrition is a significant cause of mortality, whether directly or secondary to complications (eg, infection). Among children with certain illnesses, failure to thrive is an independent risk factor for premature mortality, such as with HIV infection [56] and epidermolysis bullosa. [57]
Complications
Aside from the unfortunate children who live so far from the protective mechanisms of the developed world, psychosocial failure to thrive is almost always recognized early enough to be completely reversed. In the developing world, or regions of the developed countries with extreme poverty and isolation, chronic unaddressed malnutrition results in permanent deficits in stature and IQ, even when weight losses can be restored. Similarly, for the child with devastating or inadequately treated organic illnesses, long-term failure to thrive can compromise final height. Malnutrition, if dramatic enough, can contribute to secondary immune deficiency and intercurrent illnesses.
-
Failure of growth in weight, length, and head circumference starting at birth, suggesting an organic etiology that occurred in utero.
-
Growth failure in length and weight with a normal head circumference in an infant with growth hormone deficiency.
-
Acquired hypothyroidism.
-
Constitutional delay of growth.
-
Failure to thrive secondary to caloric deprivation.









