Background
Respiratory alkalosis is one of many acid-base disorders found among critically ill patients. It is detected by arterial blood gas (ABG) analysis and electrolyte levels. To diagnose respiratory alkalosis or assess the severity of the condition, the physician must understand clinical acid-base balance. Alkalosis, by definition, is a pathologic state that causes or tends to cause an increase in blood pH. Hence, one can have an alkalosis with normal pH if compensation has occurred; alkalemia is defined as a blood pH above 7.45. The term respiratory in respiratory alkalosis refers to the primary respiratory mechanism responsible for the change. [1]
Pathophysiology
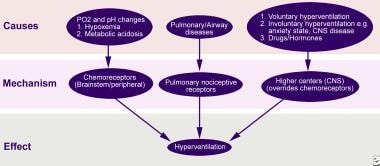
Hypocapnia (low PCO2) develops whenever CO2 elimination by the lungs exceeds tissue production. One or more of three basic categories usually underlie respiratory alkalosis: hypoxia, pulmonary diseases, and CNS disorders [2] (see image below).
Compensation
In respiratory acid-base disturbances, changes in ventilation, and hence PCO2, represent the primary disturbance, and compensation occurs by alterations in plasma bicarbonate.
In chronic respiratory alkalosis, increased urinary bicarbonate excretion resists the pH change caused by hypocapnia. This renal compensation begins within several hours and takes several days for the maximal response.
In acute respiratory alkalosis, an initial small decrease may occur in plasma bicarbonate concentration because of chemical mass action. [3] Hypocapnia leads to increased formation of carbonic acid, to lowered plasma hydrogen ion concentration (alkalemia), and to concomitant reduced plasma bicarbonate concentration. This is quantitatively less profound than renal compensation and is not related to change in bicarbonate excretion. [4]
Formulas for estimating appropriate compensation in simple respiratory alkalosis (limit of compensation is [HCO3-] of approximately 15) include the following:
-
Acute alkalosis - Change in pH = (change in PCO2) X 0.08
-
Chronic alkalosis - Change in pH = (change in PCO2) X 0.003
-
Acid-base nomogram shows confidence bands for simple acid-base disturbances. Conversion factor is 1 torr = 0.13 kPa.
-
Schematic presentation of pathophysiology of hyperventilation.