Background
Like many other lesions associated with congenital heart disease (CHD), the terminology that surrounds double-chambered right ventricle (DCRV) has evolved over the past several decades. Double-chambered right ventricle was originally described more than 130 years ago. Clinical series began describing it extensively in the 1960s.
Double-chambered right ventricle is better understood as a form of septated right ventricle (RV) caused by the presence of abnormally located or hypertrophied muscular bands.
The abnormally located or hypertrophied muscle bundles divide the RV cavity into a proximal and a distal chamber. Those muscle bundles run between an area located in the ventricular septum, beneath the level of the septal leaflet of the tricuspid valve, and the anterior wall of the RV. Frequent associated lesions include ventricular septal defect (VSD), pulmonary valve stenosis, and discrete subaortic stenosis. [1]
As outlined by Restivo et al, several subtypes of divided RV are noted. [2] These subtypes include anomalous septoparietal band, anomalous apical shelf, hypertrophy of apical trabeculations, anomalous apical shelf with Ebstein malformation, and sequestration of the outlet portion of the ventricle from a circumferential muscular diaphragm in patients with tetralogy of Fallot. Double-chambered right ventricle, the most common form, is noted by the presence of anomalous muscle bundles (AMB) that divide the RV into two chambers. However, no uniformity is observed in the position of these anomalous muscle bundles or in the manner in which the RV is divided.
See the images below.
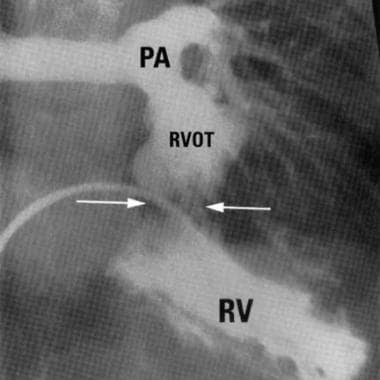 Double-Chambered Right Ventricle. Right anterior oblique (RAO) angiogram demonstrating proximal and distal chambers of right ventricle (Image courtesy of R.M. Freedom, MD).
Double-Chambered Right Ventricle. Right anterior oblique (RAO) angiogram demonstrating proximal and distal chambers of right ventricle (Image courtesy of R.M. Freedom, MD).
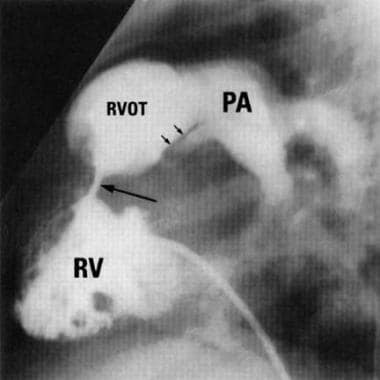 Double-Chambered Right Ventricle. Lateral right ventriculography of a patient with double-chambered right ventricle. Large arrow indicates the presence of a fibromuscular obstruction with division of the right ventricle; small arrows outline pulmonary valve stenosis (Image courtesy of R.M. Freedom, MD).
Double-Chambered Right Ventricle. Lateral right ventriculography of a patient with double-chambered right ventricle. Large arrow indicates the presence of a fibromuscular obstruction with division of the right ventricle; small arrows outline pulmonary valve stenosis (Image courtesy of R.M. Freedom, MD).
Pathophysiology
Anomalous muscle bundles divide the right ventricle (RV) into a high-pressure proximal chamber and a lower-pressure distal chamber. Evidence suggests that double-chambered right ventricle is an acquired disorder in those patients with appropriate substrate. Obstruction to pulmonary blood flow usually progresses with hypertrophy of the muscle and further obliteration of the RV cavity, although cases without progression of obstruction and even of spontaneous regression have been described.
The origin of anomalous muscle bands has been debated. The embryologic basis for double-chambered right ventricle was attributed to failure to incorporate bulbus cordis into the RV or an elevated hypertrophied moderator band. However, Byrum et al used the pattern of electrical activation to determine that muscle bundles were not the result of a displaced moderator band and suggested that activation of the double-chambered right ventricle is similar to activation of the normal heart. [3] Others, however, concluded that both the presence of bundle branch block in some patients and detection of a portion of the right bundle branch in a pathologic sample of the muscle bundle have proven the hypothesis that the moderator band is, in fact, the obstructing bundle.
A contemporary analysis of the origin of the muscle bundles determined the muscular shelf originates from the body of the septomarginal trabeculation. Two positions of muscle bundles are described as high (or horizontal) position and low (or oblique) position. Either position of the shelf divides the apical trabeculated RV in two. This same analysis determined that the normal moderator band widely varies and that the anomalous muscle bundles do not represent an early takeoff from the moderator band in most cases. In a review of surgical cases, 45% of cases had more than one or nondiscrete muscle bundles. [4]
Muscle bundles and the RV itself are usually lined with thickened endothelium. Other, less common, forms of divided RV include those in which a fibromuscular diaphragm or atrioventricular valve tissue partition the RV. These other forms include the anomalous septoparietal band, anomalous apical shelf, hypertrophy of apical trabeculations, anomalous apical shelf with Ebstein malformation, and sequestration of the outlet portion of the ventricle from a circumferential muscular diaphragm in patients with tetralogy of Fallot. These forms are not discussed in this article.
Associated defects are present in approximately 80-90% of patients; a ventricular septal defect (VSD) that involves the membranous septum is the most common defect described. A VSD may communicate with either the proximal or distal chamber, leading to a greater shunt in the latter situation. Development of RV outflow tract obstruction occurs in 3-7% of patients with membranous VSDs within the first years of life. The mechanism responsible for acquired RV obstruction may be progressive hypertrophy and obstruction from anomalous RV muscle bundles.
A well-known relationship is described among patients with RV outflow tract obstruction, membranous VSD, and subaortic stenosis. Vogel et al described 36 patients with membranous VSD and double-chambered right ventricle, 88% of whom had echocardiographic evidence of subaortic stenosis, with evidence of progressive left ventricular outflow tract obstruction. [5] Progression of subaortic stenosis may occur before or after VSD closure and/or muscle bundles are resected.
The next most common associated lesion is pulmonary valve stenosis. Various other associations have been reported, including double outlet RV, tetralogy of Fallot, anomalous pulmonary venous drainage, complete or corrected transposition of great arteries, pulmonary atresia with intact ventricular septum, and Ebstein anomaly. Double-chambered right ventricle has also been reported in patients with Down syndrome and Noonan syndrome, although differentiation from hypertrophic cardiomyopathy in the latter group is not straightforward.
Although Rowland et al considered patients in 4 groups, based on predominant physiology (pulmonary stenosis, tetralogy of Fallot, large VSD with left-to-right shunt, double-chambered right ventricle associated with other more hemodynamically significant lesions), most patients have moderate-to-restrictive VSD. [6] Most of the remaining patients present with tetralogy physiology or have significant associated lesions.
Natural history varies depending on the presence of associated lesions. Progressive obstruction of the RV outflow tract has been observed and can lead to RV failure, especially in the presence of a VSD. Several report diagnosis in asymptomatic adults in whom anomalous muscle bundles and intact ventricular septum may have been associated with a VSD that underwent spontaneous closure.
Etiology
No inheritance pattern has been described, and no risk factors for developing the disease have been encountered.
Sporadic cases have been described in patients with Down syndrome and Noonan syndrome.
Epidemiology
International data
Double-chambered right ventricle is relatively rare as an isolated anomaly; a large pediatric center typically diagnoses fewer than 10 cases per year. The lesion makes up approximately 0.5-2% of congenital heart disease and occurs in as many as 10% of patients with ventricular septal defect. [7]
Sex- and age-related data
Male-to-female ratio is 2:1. [1]
Presentation can be as early as the newborn period; however, mean age at diagnosis is in early childhood. Both fetal and adult cases have been reported.
-
Double-Chambered Right Ventricle. Electrocardiogram of an 18-month-old boy with double-chambered right ventricle. Note the upright T waves in the right precordial leads.
-
Double-Chambered Right Ventricle. Right anterior oblique (RAO) angiogram demonstrating proximal and distal chambers of right ventricle (Image courtesy of R.M. Freedom, MD).
-
Double-Chambered Right Ventricle. Lateral right ventriculography of a patient with double-chambered right ventricle. Large arrow indicates the presence of a fibromuscular obstruction with division of the right ventricle; small arrows outline pulmonary valve stenosis (Image courtesy of R.M. Freedom, MD).
-
Double-Chambered Right Ventricle. Subcostal right anterior oblique (RAO) echocardiograph view demonstrating right ventricle muscle bundles separating proximal from distal (*) chamber. PV = Pulmonary valve (Image courtesy of J. Smallhorn, MD)
-
Double-Chambered Right Ventricle. Subcostal right anterior oblique (RAO) echocardiograph view with color Doppler demonstrating ventricular septal defect jet to proximal chamber. (*) = Distal chamber (Image courtesy of J. Smallhorn, MD).







