Practice Essentials
Diphyllobothriasis is a parasitic infection caused by cestodes of the genus, Diphyllobothrium, also known as “broad tapeworms” or “fish tapeworms”. [1] There are numerous species of Diphyllobothrium, the commonest being Diphyllobothrium latum, with others including Diphyllobothrium nihonkaiense, Diphyllobothrium dendriticum, and Adenocephalus pacificus, (syn. Diphyllobothrium pacificum), being less common. [2, 3, 4] Humans are the definitive host, infected by ingestion of raw fish containing the larval forms of the cestode that evolve into the adult worm finally residing in the intestines. Although the manifestations may not be life-threatening, patients typically present with symptoms of diarrhea, abdominal discomfort, vitamin B12 deficiency, or rarely, intestinal obstruction. [1] Through globalization, human migration, and increased consumption of raw fish, the disease that was traditionally limited to the circumpolar areas of the world, has re-emerged with increased prevalence in other regions, stimulating new interest and research in its prevention.
Signs and symptoms
Most infections with diphyllobothriasis are asymptomatic. [1] In symptomatic persons, the following are the most common symptoms:
-
Diarrhea
-
Abdominal pain or discomfort
-
Constipation
-
Fatigue
-
Passage of proglottids
-
Headache
-
Allergic reactions
Prolonged infection can lead to low vitamin B12 levels in 40% of infected individuals, as the parasite leads to dissociation of the vitamin B12-intrinsic factor complex in the intestinal lumen and subsequently absorbs most of the B12. Many patients with diphyllobothrasis have no signs of illness. [1] Rare physical findings (likely related to vitamin B12 deficiency) that may be noted include the following:
-
Pallor
-
Glossitis
-
Disturbances of movement and coordination, loss of vibratory sense and proprioception
Rarely, infection with this parasite can lead to obstruction of lumens, resulting in intestinal obstruction, cholangitis, appendicitis and cholecystitis. [1, 5]
See Presentation for more detail.
Diagnosis
Laboratory studies that may be helpful for diphyllobothriasis include the following:
-
Microscopic stool examination for ova and parasites (the usual basis for the diagnosis)
-
Complete blood count (CBC) demonstrating anemia
-
Mean cell volume demonstrating macrocytosis
-
Peripheral smear demonstrating macrocytosis (unless also iron deficient) and hypersegmented polymorphonuclear neutrophils
-
Vitamin B12 level of less than 150 pmol/L and total serum homocysteine level of more than 13 µmol/L or methylmalonic acid level of more than 0.4 µmol/L in the absence of renal failure and folate and vitamin B-6 deficiencies. Vitamin B12 deficiency is reported more frequently in D latum infections than with other species. [6, 7]
Other studies that may be considered are as follows:
-
Diagnostic imaging modalities - Not typically required, except as clinically indicated by other aspects of the presentation (eg, obstruction); abdominal ultrasonography, a real-time, non-invasive test might detect the hyperechoic, ribbon-like structure freely floating in the intestinal lumen. [8]
See Workup for more detail.
Management
Most patients with diphyllobothriasis, unless they have severe symptoms, can be safely treated as outpatients.
Treatment of the infection is pharmacologic, involving one of the following agents:
-
Praziquantel (drug of choice) 5-10 mg/kg orally in single dose; taken with liquids during a meal
-
Niclosamide (acceptable alternative, but not available in the United States)
If the first course of treatment fails, a second identical course of therapy may be administered.
Other aspects of treatment include the following:
-
Surgical treatment is not required unless otherwise indicated (eg, intestinal obstruction).
-
Vitamin supplementation may be required in severe cases of vitamin B12 deficiency.
-
As a rule, no activity limitations or restrictions are necessary.
See Treatment and Medication for more detail.
Background
Diphyllobothriasis is defined as human intestinal infection with the cestode D latum, D nihonkaiense, or other broad tapeworm species. It is acquired by ingestion of inadequately cooked or unfrozen freshwater, anadromous, or marine fish containing larvae called plerocercoids, either in fish muscle or on serosal surfaces. [1] Most individuals with diphyllobothriasis have minimal or minor gastrointestinal symptoms or present with passage of worm segments (ie, proglottids) in stool.
Adult D latum is the largest human parasite and can grow to a length of greater than 20 meters and live for decades. It is capable of causing vitamin B12 deficiency through dissociation of the vitamin from intrinsic factor and consumption of the vitamin. [13] This is rare today, but low vitamin B12 levels have been reported in up to 40% of patients with D latum [1] infestation and 5% of patients with A pacificus infestation. [2] Clinical anemia and neurological disease is much rarer (< 2%). [1]
Evidence of human A pacificus infestation and D latum infestation dates to at least 6,000 years ago in Peru and Germany, respectively, and D nihonkaiense to 1,000 years ago in Japan, [14] with the first clinical description in Switzerland in 1592. Diphyllobothriasis’s relationship to fish was first noted by Sporing in 1747. The disease appears to have been particularly widespread in Baltic and Alpine freshwater areas up through the early 20th century. [1]
In the early 1970s, an estimated 9 million people were infested with D latum worldwide, with 5 million in Europe, 4 million in Asia, and the remainder in the Americas. [1]
The disease is notable for its association with temperate climate and rarity in the tropics.With improvement in human sewerage treatment, prevalence has significantly decreased in developed areas; however, some zoonotic infection persists, especially with species other than D latum, owing to nonhuman definitive hosts, including bears, wolves, marine birds, and sea lions.
With the increased popularity of raw seafood as part of ethnic foods, wider and faster food distribution networks, and, possibly, fish aquaculture, diphyllobothriasis seems to be extending its traditional geography and resurging, particularly with species other than D latum. [3, 15]
Pathophysiology
Fish tapeworms have a complex life cycle, with humans, other fish-eating mammals, and birds as their definitive hosts.
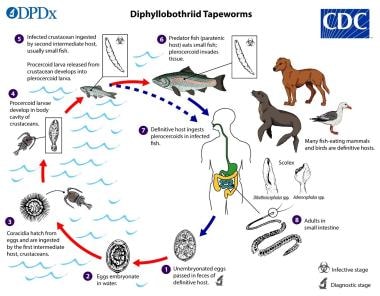 Diphyllobothrium life cycle. This illustration depicts the life cycle of different species of Diphyllobothrium parasitic cestodes, the causal agents of the disease diphyllobothriasis. Courtesy of the Centers for Disease Control and Prevention (CDC) (https://www.cdc.gov/dpdx/diphyllobothriasis/).
Diphyllobothrium life cycle. This illustration depicts the life cycle of different species of Diphyllobothrium parasitic cestodes, the causal agents of the disease diphyllobothriasis. Courtesy of the Centers for Disease Control and Prevention (CDC) (https://www.cdc.gov/dpdx/diphyllobothriasis/).
Adults are long ribbonlike creatures originally identified based on scolex and egg morphology; increasingly, cytochrome oxidase (COX1) molecular testing has clarified nosology and epidemiology. [15] Except for D latum, and possibly D nihonkaiense, which appears specifically adaptive to humans as the preferential host and are by far the most common species involved in human infection, most other species rely on non-human definitive hosts, and humans are incidentally infected. [15]
A full-grown Diphyllobothrium worm is the longest human tapeworm; it can range from 1-15 m in length and 1-2 cm in width. [16] It consists of up to 3000-4000 proglottids. The scolex has 2 sucking grooves, also called bothria, that attach to small-intestinal mucosa. Proglottids are typically wider than they are long and contain hermaphroditic sexual parts. Humans and other animals may be infested with multiple worms simultaneously.
In the gravid state, the worms have a distinctive rosettelike uterus in the center. Each adult worm sheds around one million operculated eggs every day. To complete their maturation, the eggs must reach water to be eaten by one of 40 species of crustacean copepods and cyclops, within which the procercoid matures.
Copepods that contain the procercoid are then eaten by small fish that function as the second intermediate host. Here, the procercoid matures into the plerocercoid in fish muscle or, in the case of D dendriticum and A pacificus, a serosal body surface such as the peritoneum or an organ such as the liver. [2, 4]
Fish surveys of infected lakes can be performed, monitoring risk via inspection of fish muscle or organs for encysted plerocercoids. [17] A study of fish caught in Lake Como, Italy, demonstrated infection rates of D latum in pike (Esox lucius) and perch (Perca fluviatilis) of 84% and 25%, respectively. This prevalence was attributed to the popularity of a local dish and the breakdown of sewerage effectiveness. [18]
Progressively larger fish that are paratenic hosts (hosts in which the larvae do not mature further) consume the infected fish. In this manner, the plerocercoid are passed on until, finally, the fish is consumed by a human, the definitive host. Over the subsequent 2-4 weeks, the plerocercoid larva attaches to the host intestine and matures into an adult that can live for up to 20 years.
Table 1. Prevalence of Diphyllobothriasis Infections (Open Table in a new window)
Species |
Geography |
Fish |
Definitive Host |
Human Prevalence |
D latum |
Finland, Baltic States, Danube Delta, Karelia, Manitoba, Great Lakes |
Perch, pike, burbot |
Humans, dogs, wolves |
Millions |
D nihonkaiense |
North Pacific Coast, Japan, Korea, East Russia |
Salmon (cherry, chum, pink, sockeye) |
Brown and black bears, wolves, dogs, foxes, mink |
Thousands (March-June peak) |
D dendriticum |
Lake Baikal region, Subarctic North America, Russia, Rocky Mountains, Patagonia |
Whitefish, salmonids |
Gulls, bears, otter |
Thousands (600/year mostly) |
A pacificus |
Peru, Chile, Ecuador, Sakhalin Island |
Marine fish (bonito, mackerel, lorna drum, croaker) |
Sea lions, seals |
Thousands |
D balanopterae |
Japan, Spain, Norway, Korea |
Anchovy, sardines, skipjack tuna |
Whales |
Hundreds (June-July peak) |
Plerocercoid larvae infect humans who have ingested infected and inadequately cooked or unfrozen fish. Because of the requirement for intermediate hosts, direct human-to-human transmission does not occur; therefore, no isolation measures are required.
Most humans with diphyllobothriasis (all forms) are asymptomatic, and the most common presentation is passage of worm parts, which can be alarming. Many patients retrospectively report diarrhea or may present with symptoms of obstruction [19] or even appendicitis. [5] Both vitamin B12 deficiency and iron deficiency anemia have been attributed to infestation with D latum, but not as often with other species. [1] Because of this, infection might be a concern for pregnant women. [20]
Etiology
Diphyllobothriasis is caused by ingestion of raw, undercooked, or unfrozen infected fish and subsequent intestinal infection. The main causative organisms are D latum and D nihonkaiense, but other Diphyllobothrium and similar species have also been reported as infecting agents, albeit much less frequently. Examples include the following [3] :
-
Diphyllobothrium dendriticum
-
Adenocephalus pacificus
-
Diphyllobothrium balanopterae
-
Diphyllobothrium alascense
-
Diphyllobothrium cameroni
-
Diphyllobothrium cordatum
-
Diphyllobothrium dalliae
-
Diphyllobothrium elegans
-
Diphyllobothrium lanceolatum
-
Diphyllobothrium orcini
-
Diphyllobothrium scoticum
-
Diphyllobothrium stemmacephalum
-
Diphyllobothrium ursi (possible synonym of D dendritum)
-
Ligula intestinalis
-
Schistocephalus solidus
Epidemiology
Diphyllobothriasis has been traditionally considered an endemic disease of specific locations where consumption of uncooked fish is common, such as Finland, Scandinavia, alpine Europe, North American lakes (D latum), northern Japan (D nihonkaiense), and Peru (A pacificus). With improvements in sanitation and understanding of disease pathogenesis, the prevalence of infection in endemic areas appears to have fallen dramatically during the 20th century. [1] Although Japan has mandated case reporting since 2012, little is known about the current prevalence and incidence of various fish tapeworm infections except for case reports and occasional surveys. [21]
Increasing travel, migration, and popularity of ethnic foods have contributed to a broadening of interest and, possibly, exposure to raw fish delicacies, particularly in Asia, [22] but also elsewhere owing to rapid food transport and fish aquaculture. [23]
Examples of foods responsible for infection include raw and salted or marinated fish filets in Scandinavia, Jewish gefilte fish, Italian carpaccio, French tartare maison (raw salmon) and poisson du lac façon nordique, Japanese sushi (raw fish on rice cakes or balls) and sashimi (sliced raw fish), and ceviche (lightly marinated fish) in Latin America. The quality and type of fish, as well as other animal products in dishes, varies by locale and cost, with the highest risk apparently associated with rural restaurants and street vendors. Types of fish and risk may vary by season. The peak risk for D nihonkaiense is associated with the spring catch of cherry and immature chum salmon in Northern Japan. The peak risk for D balanopterae in Northern Japan is associated with the harvest of anchovies from June to August. [21]
Concerns have been raised regarding salmon and other fish aquaculture, particularly in Chile, because of growing salmon smolt in lakes endemic for D latum and potential contamination and escape of infected fish grown in oceanic pens. [23] Such infection might account for infections with D latum in Brazil [24] and other tropical areas such as Taiwan [25] and South India. [26] Similarly, infection with A pacificus in Spain might be associated with heavy importation of chilled fish from Chile, Ecuador, and Peru. [2, 27] Although salmonids are not the preferred intermediate host for D latum in the Northern hemisphere, recent reports of D latum plerocercoid larvae adapting to salmonids are especially alarming in South America and may have important epidemiologic consequences as the consumption of salmonids increases. [28] Placing fish on ice for transport is inadequate to kill plerocercoids and therefore, adequate freezing of fish and parasitologic screening before exportation may be warranted to prevent the spread of disease. [28]
In addition to human migration and importation of disease, other factors might account for the increased incidence and range of disease. These include, in the case of D dentriticum, migrating birds such as gulls around Lake Baikal and climate change affecting intermediate hosts. [4] In the absence of effective surveillance and reporting, these diseases will primarily be discovered incidentally. Fish surveys show variable positivity, with D latum plerocercoid larvae in 84% of pike in Lake Como in 2013-2014 [18] and 51% of Tokyo chum salmon in 2001[14] to 20% of various species of Peruvian marine fish infested with A pacificus in 2014.[2]
Workers in the fish industry may be at some risk for diphyllobothriasis, particularly for serosal or organ-encysted worms such as D dendriticum and A pacificus, from gutting and either contamination or habits of consumption of roe and fish organs while working. [2, 4]
Diphyllobothriasis has no reported age predilection or sexual predilection. In addition, it has no known racial predilection, except as would be expected based on geographic and cultural factors.
Prognosis
Diphyllobothriasis carries an excellent prognosis. D latum is not invasive, and mortality due to diphyllobothriasis is rare. Patients are often frightened and emotionally upset at the passage of worm parts for a long period of time unless treated. Single-dose therapy is usually effective, although some treatment failures have been reported, and repeat treatment is occasionally needed.
Occasionally, infestation can lead to severe megaloblastic anemia or intestinal obstruction. Although it is well described, macrocytic anemia and neurological disease is extremely rare.Gastrointestinal (GI) obstruction is also rare but may occur, especially when numerous worms are present.
Patient Education
Because reinfection is possible, patients should be advised to modify their dietary habits to minimize the potential for reexposure.
Proper food preparation and hygiene should be encouraged, particularly during travel within endemic areas. Preventive public education has been generally inadequate, and the food and restaurant industry has generally been slow to adopt recommendations for freezing of fish. Simple icing of fish is insufficient to prevent infection.
The US Centers for Disease Control and Prevention (CDC) recommends cooking fish to an internal temperature of 63°C (145°F) or higher, freezing fish to -4°F (-20°C) for 7 days or -31°F (-35°C) or less until solid, and storing at either -31°F (-65°C) or below for 15 hours or -4°F (-20°C) for 24 hours. [29] These precautions kill the plerocercoid larvae. The fish must never be sampled before it is properly prepared.
-
Life cycle of diphyllobothrium.
-
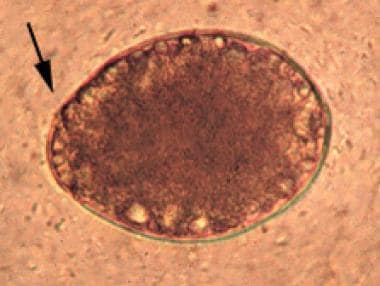
Egg of Diphyllobothrium latum with arrow pointing to operculum.
-
Proglottids of Diphyllobothrium latum.
-
Diphyllobothrium life cycle. This illustration depicts the life cycle of different species of Diphyllobothrium parasitic cestodes, the causal agents of the disease diphyllobothriasis. Courtesy of the Centers for Disease Control and Prevention (CDC) (https://www.cdc.gov/dpdx/diphyllobothriasis/).