Practice Essentials
Human cytomegalovirus (CMV) is 1 of 8 human herpesviruses. It is a member of the beta-herpesvirus subfamily, which also includes the roseolaviruses, human herpesvirus type 6, and human herpesvirus type 7. [1]
Infection with CMV is ubiquitous and generally asymptomatic in healthy children and adults. However, several high-risk groups, including immunocompromised organ transplant recipients and individuals infected with human immunodeficiency virus (HIV), are at risk of developing life-threatening and sight-threatening CMV disease. CMV is also a major cause of morbidity and occasional mortality in newborn infants. In recent years, it has become evident that CMV is the most important cause of congenital infection in the developed world, and that it frequently leads to intellectual and developmental disability. In addition, increasing evidence suggests that CMV may cause long-term health consequences in healthy adults, including immunosenescence and an increased risk of malignancy and vascular disease. [2] Risk factors for cytomegalovirus (CMV)–associated illness chiefly include age and immunodeficiency.
Signs and symptoms
Physical examination findings depend on age, route of acquisition, and immune status of the patient.
About 10% of infants with congenital infection have clinical evidence of disease at birth. The most severe form of congenital CMV infection is referred to as cytomegalic inclusion disease, which is characterized by intrauterine growth restriction; hepatosplenomegaly; hematologic abnormalities (particularly thrombocytopenia); various cutaneous manifestations, including petechiae and purpura; and neurologic manifestations, such as microcephaly, ventriculomegaly, cerebral atrophy, chorioretinitis, and sensorineural hearing loss.
See Presentation for more detail.
Diagnosis
Laboratory studies
Viral culture is the most important diagnostic study in the evaluation of suspected CMV disease. CMV may be cultured from virtually any body fluid or organ system.
Polymerase chain reaction and CMV antigenemia studies have emerged as the studies of choice in monitoring the status of CMV replication and establishing the diagnosis of CMV disease in immunocompromised patients.
Imaging studies
The most important study in the diagnostic evaluation of the congenitally infected infant with CMV is head computed tomography (CT) scanning (see the image below).
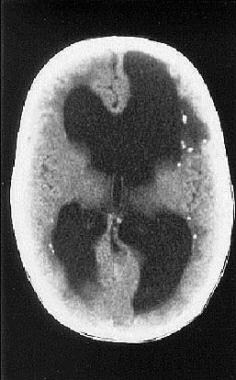 Cranial CT scan of infant born with symptomatic congenital cytomegalovirus infection. Neurological involvement is evident, manifest as ventriculomegaly and periventricular calcifications.
Cranial CT scan of infant born with symptomatic congenital cytomegalovirus infection. Neurological involvement is evident, manifest as ventriculomegaly and periventricular calcifications.
See Workup for more detail.
Management
Medical care of patients with CMV infection consists of good nutritional support, vigorous supportive care for end-organ syndromes (particularly pneumonia in immunocompromised patients), and specific antiviral therapy in select circumstances.
See Treatment and Medication for more detail.
Background
In 1904, Ribbert first identified histopathological evidence of CMV, probably in tissues from a congenitally infected infant. Ribbert mistakenly assumed that the large inclusion-bearing cells he observed at autopsy were from protozoa (incorrectly named Entamoebamortinatalium). In 1920, Goodpasture correctly postulated the viral etiology of these inclusions. [3] Goodpasture used the term cytomegalia to refer to the enlarged, swollen nature of the infected cells. Human CMV was first isolated in tissue culture in 1956, and the propensity of this organism to infect the salivary gland led to its initial designation as a salivary gland virus.
In 1960, Weller designated the virus cytomegalovirus [4] ; during the 1970s and 1980s, knowledge of the role of CMV as an important pathogen with diverse clinical manifestations increased steadily. [5] Although enormous progress has recently been made in defining and characterizing the molecular biology, immunology, and antiviral therapeutic targets for CMV, considerable work remains in devising strategies for prevention of CMV infection and in understanding the role of specific viral genes in pathogenesis.
Furthermore, development of a vaccine against this virus is a major public health priority (reviewed in Prevention). [6, 3, 7]
Pathophysiology
As noted, cytomegalovirus (CMV) is a member of a family of 8 human herpesviruses, officially designated as human herpesvirus type 5 (HHV-5). Taxonomically, CMV is referred to as a Betaherpesvirinae, based on its propensity to infect mononuclear cells and lymphocytes and on its molecular phylogenetic relationship to other herpesviruses. CMV is the largest member of the herpesvirus family, with a double-stranded DNA genome of more than 240 kbp, capable of encoding more than 200 potential protein products. The function of most of these proteins remains unclear. As with the other herpesviruses, the structure of the viral particle is that of an icosahedral capsid, surrounded by a lipid bilayer outer envelope.
An understanding of the process of viral replication provides insights into molecular mechanisms of antiviral therapy and protective immunity. The relationship between viral replication and the pathogenesis of infection is the subject of a recent review. [8] CMV replicates very slowly in cell culture, mirroring its very slow pattern of growth in vivo (in contrast to herpes simplex virus [HSV] infection, which progresses very rapidly). The replication cycle of CMV is temporally divided into the following 3 regulated classes: immediate early, early, and late.
Immediate early gene transcription occurs in the first 4 hours following viral infection, when key regulatory proteins that allow the virus to take control of cellular machinery are made. The major immediate early promoter of this region of the CMV genome is one of the most powerful eucaryotic promoters described in nature; this has been exploited in modern biotechnology as a useful promoter for driving gene expression in gene therapy and vaccination studies.
Following the synthesis of immediate early genes, the early gene products are transcribed. Early gene products include DNA replication proteins and some structural proteins.
The late gene products are made approximately 24 hours after infection, and these proteins are chiefly structural proteins that are involved in virion assembly and egress. Synthesis of late genes is highly dependent on viral DNA replication and can be blocked by inhibitors of viral DNA polymerase, such as ganciclovir. The lipid bilayer outer envelope contains the virally encoded glycoproteins, which are the major targets of host neutralizing antibody responses. These glycoproteins are candidates for human vaccine design. The proteinaceous layer between the envelope and the inner capsid, the viral tegument, contains proteins that are major targets of host cell–mediated immune responses. The most important of these tegument proteins is the so-called major tegument protein, UL83 (phosphoprotein 65 [pp65]).
Another clinically important gene product, the UL97 gene product, is a phosphotransferase. Although the function of this protein in the viral life cycle is unknown, this gene is clinically important because a substrate of the kinase is the antiviral drug ganciclovir, which, once phosphorylated, becomes a highly effective CMV therapy. [9]
In clinical specimens, one of the classic hallmarks of CMV infection is the cytomegalic inclusion cell. These strikingly enlarged cells (the property of "cytomegaly," from which CMV acquires its name) contain intranuclear inclusions that have the histopathological appearance of owl's eyes. The presence of these cells indicates productive infection, although they may be absent even in actively infected tissues. In most cell lines, CMV is difficult to culture in the laboratory; however, in vivo infection seems to chiefly involve epithelial cells. In severe disseminated CMV disease, involvement can be observed in most organ systems.
Little is known about the molecular mechanisms responsible for the pathogenesis of tissue damage caused by CMV, particularly for congenital CMV infection. The mechanisms by which CMV injures the fetus are complex and likely include a combination of direct fetal cellular injury (particularly in the fetal brain) induced by pathologic virally encoded gene products, an incomplete maternal immune response incapable of fully controlling infection, and the impact of infection on placental function, including oxygen and substrate transport.
CMV also encodes gene products that function, at both the RNA and the protein level, to interfere with many cellular processes. These include gene products that modify the cell cycle; gene products that interfere with apoptosis; functions that induce an inflammatory response; gene products that mediate vascular injury; proteins that induce site-specific breakage of chromosomes; gene products that promote oncogenesis and dysregulation of cellular proliferation; and, most strikingly, genes that facilitate evasion of host immune responses. [8]
Immunity to CMV is complex and involves humoral and cell-mediated responses. Several CMV gene products are of particular importance in CMV immunity. The outer envelope of the virus, which is derived from the host cell nuclear membrane, contains multiple virally encoded glycoproteins. Glycoprotein B (gB) and glycoprotein H (gH) appear to be the major determinants of protective humoral immunity. Antibody to these proteins is capable of neutralizing virus, and gB and gH are targets of investigational CMV subunit vaccines; however, although humoral responses are important in control of severe disease, they are clearly inadequate in preventing transplacental infection, which can occur even in women who are CMV seropositive.
CMV uses 2 pathways of entry into the cell: (1) a fusion-mediated pathway in fibroblasts and (2) an endocytosis-mediated pathway in epithelial and endothelial cells. CMV proteins that are important in the endocytosis-mediated entry pathway, encoded by the UL128-131 genes, may emerge as particularly useful vaccine candidates in future studies. [10, 11]
The generation of cytotoxic T-cell (CTL) responses against CMV may be a more important host immune response in control of infection. In general, these CTLs involve major histocompatibility complex (MHC) class I restricted CD8+ responses. Although many viral gene products are important in generating these responses, [12] most CMV-specific CTLs target an abundant phosphoprotein in the viral tegument, pp65, the product of the CMV UL83 gene. In passive transfer experiments involving high-risk bone marrow transplant recipients, the value of these responses was dramatically demonstrated using adoptive transfer of CMV-specific CD8+ T cells that target the CMV UL83 gene, which was able to control CMV disease. [13]
Investigations into the molecular biology of CMV have revealed the presence of many viral gene products, which appear to modulate host inflammatory and immune responses. [14] Several CMV genes interfere with normal antigen processing and generation of cell-mediated immune responses, including the US11 gene product, which exports the class I heavy chain from the endoplasmic reticulum (ER) to the cytosol (rendering it nonfunctional). Another is the US3 gene product, which retains MHC molecules in the ER, preventing them from traveling to the plasma membrane. Finally, the US6 protein inhibits peptide translocation by transporters associated with antigen processing (TAP).
Other viral gene products, the UL33, US27, and US28 genes, are functional homologs of cellular G-protein coupled receptors, which may, via molecular mimicry, subvert normal inflammatory responses and, in the process, promote tissue dissemination of the virus and interfere with the host immune response.
The cytomegalovirus genome also encodes a homolog of the cellular major histocompatibility class I gene, which appears to contribute to the ability of CMV to evade host defense. The UL144 open reading frame found in clinical isolates of CMV encodes a structural homolog of the tumor necrosis factor receptor superfamily, and this too may contribute to the ability of human CMV to escape immune clearance. Other cytomegalovirus genes interfere with natural killer (NK) cell responses, including the UL18 gene product. A better understanding of the impact of viral immune evasion genes on the development of protective immunity to CMV infection should enable the design of improved vaccines. [15]
Epidemiology
United States statistics
Every mammal appears to be infected with its own species-specific cytomegalovirus (CMV), and no evidence suggests that infections cross species. Hence, humans are the only natural host for human CMV infection. Although most adults eventually become infected with CMV, the epidemiology of this infection is complex, and the age at which an individual acquires CMV greatly depends on geographic location, socioeconomic status, cultural factors, and child-rearing practices.
In developing countries, most children acquire CMV infection early in life, with adult seroprevalence approaching 100% by early adulthood. In contrast, in developed countries, the seroprevalence of CMV approximates 50% in young adults of middle-upper socioeconomic status. This observation has important implications for congenital CMV epidemiology because women of childbearing age who are CMV seronegative are at major risk of giving birth to infants with symptomatic congenital infection if primary infection is acquired during pregnancy.
Transmission of CMV infection may occur throughout life, chiefly via contact with infected secretions. [16] In the developed world, CMV is the most common congenital viral infection. An overall rate of congenital CMV transmission of approximately 1% (ranging from 0.25–2%, depending on the population studied) has been estimated in newborn infants in the developed world in most reviews. This translates to about 80,000 congenital CMV infections per year in the United States and Europe. In the United States, congenital CMV infection occurs in 3-6 of every 1000 infants born each year. [17]
A meta-analysis of published studies concluded that the overall birth prevalence of congenital CMV infection was 0.64%, but noted that rates varied considerably among different study populations. [18] Nonwhite race, low socioeconomic status, premature birth, and neonatal intensive care unit admittance were risk factors for congenital CMV infection; and birth prevalence increased with maternal CMV seroprevalence.
There has been insufficient attention given to congenital CMV infection in the developing world, but the limited data available suggest that CMV may also represent a significant public health concern in these populations. [19] A multicenter study comparing newborn screening by oropharyngeal culture versus polymerase chain reaction (PCR)–based detection of viral DNA in the newborn dried blood spot indentified an overall prevalence of 0.45% in the 7 sites surveyed. [20] Most congenital CMV infections occur in infants born to mothers with preexisting immunity, and these infections are clinically asymptomatic at birth; however, long-term sequelae, including deafness, can occur (see History).
The route of congenital infection is presumed to be transplacental. The transplacental transmission rate after maternal primary infection is about 32%. [21, 22] CMV may also be transmitted perinatally, both by aspiration of cervicovaginal secretions in the birth canal and by breastfeeding. More than 50% of infants fed with breast milk that contains infectious virus become infected with CMV. [23] In particular, a study reported 5 cases of severe morbidity and mortality in very low birth weight infants with CMV infection acquired postnatally through breast milk. [24] Infants who are not infected congenitally or perinatally with CMV are at high risk to acquire infection in daycare centers. According to some studies, the prevalence of CMV infection in children who attend daycare, particularly children younger than 2 years, approximates 80%.
The virus may be readily transmitted to susceptible children via saliva, urine, and fomites; these children, in turn, may transmit infection to their parents. [25, 26] Horizontal transmission of infection in daycare centers appears to play a major role in the epidemiology of many CMV infections in young parents. [27]
In adulthood, sexual activity is probably the most important route of acquisition of CMV, [28] although the observation that virus is present in saliva, cervicovaginal secretions, and semen obscures which route or routes of transmission are primarily responsible for establishment of infection. Saliva alone appears to be sufficient for transmission of CMV, and this route of transmission may be responsible for those cases of heterophile-negative mononucleosis, which are attributable to CMV. Kissing appears to be a way in which CMV is transmitted from toddlers to seronegative parents. Work by the Centers for Disease Control and Prevention (CDC) has emphasized the need for greater public awareness of these risks and for educational interventions for young women of childbearing age. [29]
Other important routes of transmission include blood transfusion and solid organ transplantation. Before screening of blood products, transfusion-associated CMV was an important cause of morbidity and mortality in premature infants; however, the routine use in many neonatal intensive care units of CMV-negative blood products has largely eliminated this problem. Posttransfusion CMV is still a risk in CMV-seronegative trauma and in surgery patients, often manifesting as hepatitis.
International statistics
The risk of congenital CMV infection is not well defined in the developing world. Because seroepidemiologic studies indicate that in many developing countries, seroprevalence for CMV approaches 100% very early in childhood, little attention has been given to the question of potential morbidities in these populations.
Race-, sex-, and age-related demographics
Race
The effects of race and genetics on clinical manifestations of cytomegalovirus (CMV) infection are not well understood. In some studies in the United States, the prevalence of congenital CMV infection appears to be higher in infants born to Black women. [30] More recent studies using the National Health and Nutrition Examination Survey (NHANES) database confirm that substantial differences exist in the prevalence of CMV infection as a function of race in the United States. [25, 26]
Congenital CMV infection, indeed, should be considered a disease of health disparities. More work is required to understand the basis for the differences in the epidemiology of CMV infection in various ethnic groups in the United States.
Sex
Both sexes are equally susceptible to infection and morbidity from cytomegalovirus (CMV), although only women are at risk for transplacental transmission of infection.
Age
The annual seroconversion rate for acquisition of cytomegalovirus (CMV) infection is approximately 1%. However, 2 age groups have higher rates of acquisition of infection: toddlers who attend group daycare and adolescents. Accordingly, these represent 2 potential groups in which to implement vaccination.
Prognosis
A cohort study by Gunkel et al that assessed the neurodevelopment in 356 infants 6 years of age and younger, of which 14% had postnatal cytomegalovirus infection, reported no adverse effect on neurodevelopment in preterm children with postnatal cytomegalovirus infection. [31]
Morbidity/mortality
Cytomegalovirus (CMV) is a substantial cause of morbidity in newborns. As the most common so-called toxoplasmosis, rubella, CMV, and herpes simplex (TORCH) infection in the developed world, CMV accounts for extensive neurodevelopmental morbidity, including sensorineural deafness in infants.
CMV also accounts for substantial mortality in immunocompromised patients. Mortality due to congenital CMV infection is low (about 4% of infants). [21, 22]
Patient Education
Increased awareness of the complications of congenital cytomegalovirus (CMV) infection is needed.
With a greater educational effort, women of childbearing age can be better prepared to anticipate risk factors for CMV transmission during pregnancy. [32]
A national CMV registry provides education and support for families affected by congenital CMV infection. Contact the National Congenital CMV Disease Registry at Feigin Center, Suite 1150, 1102 Bates Street, MC 3-2371, Houston, TX, 77030-2399, (832) 824-4387, or visit the Web site at http://www.bcm.tmc.edu/pedi/infect/cmv/ .
Better education of the risks of CMV infection for young women is a must. The CDC is also an excellent educational resource.
Other foundations provide education and resources for parents interested in learning more about congenital CMV, including the CMV Foundation.
-
Epidemiology patterns of congenital cytomegalovirus infection. Approximately 10% of cases of congenital cytomegalovirus occur in women with primary infection during pregnancy, and 90% of these infants have neurological sequelae. Although preexisting immunity (eg, maternal recurrent infection) protects against severe disease, approximately 15% of these infants have sequelae, particularly sensorineural hearing loss.
-
Cranial CT scan of infant born with symptomatic congenital cytomegalovirus infection. Neurological involvement is evident, manifest as ventriculomegaly and periventricular calcifications.