Background
Coccidioidomycosis is caused by Coccidioides immitis, a soil fungus native to the San Joaquin Valley of California, and by C posadasii, which is endemic to certain arid-to-semiarid areas of the southwestern United States, northern portions of Mexico, and scattered areas in Central America and South America. Although genetically distinct, the 2 species are morphologically identical. [1, 2, 3]
Few immunologic differences are noted between the 2 species of Coccidioides, and the manifestations of infection with either organism are assumed to be identical; however, this hypothesis has not been formally tested. For all intents and purposes, all subsequent references to C immitis in this article also apply to C posadasii.
See A Chronic, Scaly Rash Mistaken for MRSA: Case Presentation, a Critical Images slideshow, to review additional images and details of a case of Coccidioidomycosis.
Clinical aspects
Coccidioidomycosis typically is transmitted by inhalation of airborne spores of C immitis or C posadasii (see Etiology). Infection occurs in endemic areas and is most commonly acquired in the summer or the late fall during outdoor activities.
Travelers to endemic areas are at risk of contracting the disease, which may not become clinically evident until after they have returned home. [4] In addition, infection may be acquired outside of endemic areas via transport of contaminated material. Diagnosis often is delayed in nonendemic areas because coccidioidal infection initially is not considered in the differential. [5, 6, 7, 8]
In most patients with coccidioidal infection, the primary infection is in the lungs. In 60-65% of cases, this infection is asymptomatic.
In other cases, a mild influenza-like illness develops 1-4 weeks after exposure. The symptoms are indistinguishable from other respiratory illnesses, with fever, sore throat, cough, headache, fatigue, and pleuritic chest pain. Resolution typically occurs over several weeks (although fatigue may persist for months), and 95% or more of patients recover without any further sequelae.
A more involved presentation, with the constellation of fever, arthralgias, erythema nodosum or erythema multiforme, and chest pain is commonly referred to as San Joaquin Valley fever (or simply Valley fever) [3] or desert rheumatism (see Workup).
Coccidioidomycosis spreads beyond the lungs in approximately 0.6% of the infections in the general population. Most extrapulmonary disseminated infections are a result of hematogenous spread. Dissemination can be rapid and fatal. Virtually any organ of the body can be involved (eg, endocrine glands, eye, liver, kidney, prostate, peritoneal cavity), but Coccidioides species has a predilection for the lungs, skin, soft tissue, joints, and CNS, especially the meninges. Meningitis is a grave complication.
Disseminated disease may occur in an otherwise healthy individual, but the risk is significantly higher in individuals with altered cellular immunity due to disease (eg, HIV infection, lymphoma), medical treatment (eg, corticosteroid therapy), or pregnancy. In addition, risk for dissemination or progressive pulmonary disease is higher in certain groups (eg, those of Oceanic or African genomic ancestry). (See Epidemiology and Clinical.)
Extrapulmonary primary infections can occur with trauma causing a puncture wound from a contaminated object. Laboratory workers and children are especially at risk for cutaneous or soft tissue lesions, including chancres, with regional lymphadenitis. [5, 6, 7, 9, 10, 11, 12]
Diagnosis requires isolation of the organism in culture, identification on histologic specimens, or serologic testing (see Workup). Most patients infected with Coccidioides are asymptomatic or have self-limited symptoms and require only supportive care. Symptomatic patients usually come to medical attention because of respiratory tract or systemic manifestations. Management in symptomatic patients varies with the clinical syndrome (see Treatment).
Amphotericin B and oral triazoles are the mainstays of antifungal therapy for coccidioidomycosis. Duration of therapy for the infection is often prolonged and may last several months to years, with lifelong suppression needed in certain patients. [5, 6, 9, 10]
Historical background
Wernicke and Posadas first described a case of coccidioidomycosis in 1892 in South America, in an Argentinean soldier with predominantly cutaneous manifestations. Two years later in the United States, a patient with disseminated coccidioidomycosis was first reported in California in 1894.
In 1896, Rixford and Gilchrist reported a few cases in which they identified the infecting agent as a protozoan-like organism and named it Coccidioides immitis. Ophuls further described the fungal life cycle and pathology of C immitis in 1905.
The disease was considered rare and uniformly fatal until 1929, when a Stanford University medical student, Harold Chope, accidentally inhaled a culture of Coccidioides and developed a nonfatal pulmonary illness accompanied by erythema nodosum. This case sparked interest that resulted in researchers uncovering the association between C immitis and the clinical condition known as San Joaquin Valley fever. Charles E. Smith and colleagues subsequently developed coccidioidin skin test and serologic testing for coccidioidomycosis.
The importance of the illness increased during the 1930s and 1940s, starting with the influx of immigrants from the Midwest who arrived in the San Joaquin Valley of California to escape drought and to seek agricultural employment. The thousands of military personnel building airstrips and participating in desert combat training during World War II led to many important studies on the pathogenic organisms and the epidemiology, clinical features, and diagnosis of coccidioidomycosis by the military health services.
Interest in coccidioidomycosis has been renewed because of massive migration to the Sunbelt states. Areas that once were sparsely populated are now major cities, which increases the population at risk for the disease. Phoenix and Tucson, Arizona; Bakersfield and Fresno, California; and El Paso, Texas, are prime examples.
These locales also have a growing population of individuals who are unusually susceptible to the most serious consequences of infection, due to advanced age or immunocompromise. Interest also has increased because of an explosion in the number of cases that occurred during the great coccidioidomycosis outbreak in California in 1991-1994.
The first effective therapy for coccidioidomycosis, intravenous amphotericin B, first was used in 1957. Since the 1980s, various oral antifungal agents, including ketoconazole, itraconazole, and fluconazole, have led to further advances in the treatment of coccidioidomycosis. The roles of newer agents (eg, voriconazole, posaconazole, isavuconazole) primarily are in the care of those with chronic or disseminated forms of coccidioidomycosis.
Pathophysiology
The vast majority of coccidioidal infections result from airborne transmission. Pulmonary infection can result from inhalation of a single spore in humans, but high inoculum exposures are more likely to result in symptomatic disease. Inhaled C immitis or C posadasii arthroconidia (ie, spores; see the image below) are deposited into the terminal bronchioles.
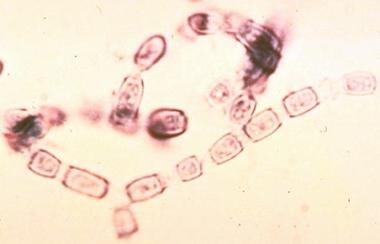 Arthroconidia become airborne and infect the human host to begin the parasitic phase of its life cycle. The arthroconidia develop into spherules containing endospores, which propagate infection in human tissues. Courtesy of Thomas Matthew.
Arthroconidia become airborne and infect the human host to begin the parasitic phase of its life cycle. The arthroconidia develop into spherules containing endospores, which propagate infection in human tissues. Courtesy of Thomas Matthew.
In the bronchioles, the arthroconidia enlarge to form spherules, which are round double-walled structures measuring approximately 20-100 μm in diameter. The spherules undergo internal division within 48-72 hours and become filled with hundreds to thousands of offspring (ie, endospores). Rupture of the spherules leads to the release of endospores, which mature to form more spherules. [7]
As an arthroconidium transforms into a spherule, the resulting inflammation results in a local pulmonary lesion. Extracts of C immitis organisms react with complement, leading to the release of mediators of chemotaxis for neutrophils.
Some of the endospores are engulfed by macrophages, initiating the acute inflammation phase. If the infection is not cleared during this process, a new set of lymphocytes and histiocytes descend on the infection site, leading to granuloma formation with the presence of giant cells. This is the chronic inflammation phase. People with severe disease may have both acute and chronic forms of inflammation.
Numerous studies have established that immunity mediated by T cells is critical to controlling the infection. [13] The innate cellular response (neutrophils, macrophages mononuclear cells, NK cells) also contributes to host defense. T-cell activation and cytokine formation stimulate inflammatory cells and facilitate killing of the organism. T-helper type 1 (Th-1) cytokines, particularly interferon-gamma, promote macrophage killing of endospores.
A failure of the host to respond appropriately indicates either a specific or a generalized deficiency in cell-mediated immunity. This is clinically overt in patients who have conditions that impair cell-mediated immunity and in those who are using agents that interfere with T-cell function. Other factors, such as immune-complex formation and antigen overload, also can cause failure of host response.
Some of the phagocytized arthroconidia are theorized to be transported back to draining lymph nodes by macrophages and can cause lymphangitis. The inoculating dose responsible for infection is small and may be 10 or fewer arthroconidia.
The presence of spherules triggers an acute inflammatory reaction. Spherules react with complement and promote chemotaxis of neutrophils and eosinophils. A mononuclear infiltrate may develop followed by subsequent conversion to polymorphonuclear predominance.
Pathogenicity of the organism largely is related to the resistance of the spherule to eradication by host defenses. Spherules and endospores produce no known toxins, and as new spherules are propagated in infected tissue, progressive suppuration and tissue necrosis occur. Neutrophils and mononuclear cells attempt phagocytosis of the organism, and giant cells are formed to attack larger fungal structures.
The body responds to the presence of the endospores with activation of complement and release of chemotactic factors. An intense, primarily neutrophilic, inflammatory reaction follows; however, the recruited neutrophils and macrophages are unable to kill the organisms because the spherules are resistant to phagocytosis. T-cell mediated immunity is important for killing and clearing of the organism; therefore, deficiencies in this arm of the immune system render the host of the fungus extremely vulnerable to disease and dissemination. [7]
Non-respiratory transmission
In rare occurrences, direct inoculation of Coccidioides (eg, from contaminated penetrating objects) causes primary cutaneous coccidioidomycosis with lymphatic extension to the regional lymph nodes; these cases resolve without treatment. In 2009, a report alleged transmission of coccidioidomycosis to a human by a cat bite. [14] This occurred in a veterinary assistant who had been bitten on the hand by a cat that was later diagnosed with disseminated disease.
Case reports have documented rare instances of coccidioidomycosis transmitted through other modes. These include transplantation of organs from infected donors and sexually transmitted cases.
Dissemination
In some individuals, Coccidioides leaves the lungs to establish disseminated lesions in distant parts of the body. To establish extrapulmonary sites of infection, the fungal elements must move from the bronchiole into the lung parenchyma and enter and leave the vascular space.
Initially, the organism may spread via lymphatic drainage of infected macrophages from the initial terminal bronchiole lesion, as demonstrated by sequential involvement of the hilar nodes, followed by the paratracheal and then supraclavicular nodes, and finally reaching the common lymphatic duct.
From the thoracic duct, spread of the infection becomes hematogenous. Certain host factors, clinical findings, and laboratory findings suggest dissemination including advanced age, immunocompromised state, late stages of pregnancy, and ethnic or racial factors (see Epidemiology).
With dissemination, cell-mediated immunity can become impaired further, often reflected by anergy to coccidioidal skin tests. The mechanism for this effect on cell-mediated immunity is unclear, although many theories have been postulated. Antigen overload, suppressor cells, formation of immune complexes, and elaboration of immunosuppressive substances by the fungi may contribute to the impairment in cell-mediated immunity. [7] Eventually, immunity may recover with treatment and control of the coccidioidomycosis.
Etiology
Coccidioidomycosis is caused by C immitis and C posadasii, 2 genetically distinct but morphologically identical species of a soil fungus endemic to certain arid-to-semiarid regions of the Western Hemisphere. The ecologic niche of Coccidioides is in the desert Southwest. This zone is characterized by low elevations (below 3700 ft), scant rainfall (5-20 in/y), mild winters (40-54°F) and hot summers, and sandy alkaline soil with increased salinity. [9, 15, 16]
Desert Southwest zones are found in areas of the Western Hemisphere from latitudes 40° north to 40° south. [5, 17, 18] The endemic areas for Coccidioides in the United States include Arizona, south central California (San Joaquin Valley), Nevada, New Mexico, certain parts of Utah, and the western half of Texas.
Other endemic areas are the regions of Mexico that border the western United States. The fungi also are endemic to some Central American countries, including Guatemala, Honduras, and Nicaragua. Certain desert regions of South America (Brazil, Argentina, Paraguay, Venezuela) also are endemic.
C immitis is largely limited to the San Joaquin Valley. C posadasii is found in the other areas of Coccidioides endemicity. The manifestations of infection with either organism are assumed to be identical.
Coccidioides life cycle
Coccidioides is a dimorphic fungus, meaning that it assumes 2 different forms, yeast or mold, depending on the environment. In soil, Coccidioides grows as a mold (mycelium) with branching septate hyphae. During the rainy season, the mycelia grow rapidly, but they also are the least infectious form of the organism. [9]
As the soil becomes drier, in late summer and early fall, the hyphae develop into arthrospores. Arthrospores measure 3-5 µm and are extremely hardy, withstanding extreme heat, desiccation, and changes in soil salinity and remaining viable in the soil for months to years. When the soil is disturbed by wind or excavation, arthrospores readily disarticulate into single arthroconidia (rectangular spores measuring 8-30 μm in diameter) and become airborne. [10] The arthroconidia are the infectious particles of coccidioidomycosis.
 Arthroconidia become airborne and infect the human host to begin the parasitic phase of its life cycle. The arthroconidia develop into spherules containing endospores, which propagate infection in human tissues. Courtesy of Thomas Matthew.
Arthroconidia become airborne and infect the human host to begin the parasitic phase of its life cycle. The arthroconidia develop into spherules containing endospores, which propagate infection in human tissues. Courtesy of Thomas Matthew.
Coccidioides arthroconidia are extremely infectious; a single C immitis arthroconidium may be sufficient to produce a respiratory infection. However, exposure to high spore burdens increases the likelihood of more severe disease in otherwise healthy persons.
If inhaled by animals or humans, the arthroconidia can reach the pulmonary alveoli. The size of the arthroconidium allows it to be deposited in the terminal bronchiole but probably does not allow it to reach the alveolar space by means of direct inhalation.
Once in the alveoli, Coccidioides enters the yeast stage of its life cycle. The arthroconidium sheds its outer coating, swells, and becomes a spherule—a round, thick-walled multinucleate structure that contains hundreds to thousands of uninucleate endospores. Rupture of the spherule leads to release of the contained endospores, each of which matures into spherules, repeating the cycle.
Rarely, direct inoculation of C immitis (eg, from contaminated penetrating objects) causes primary cutaneous coccidioidomycosis with lymphatic extension to the regional lymph nodes; these cases resolve without treatment.
Domesticated, zoo, and wild animals also can be infected with Coccidioides species. In 2009, a report alleged transmission of coccidioidomycosis to a human by a cat bite. [14] This occurred in a veterinary assistant who had been bitten on the hand by a cat that was later diagnosed with disseminated disease.
Case reports have documented rare instances of coccidioidomycosis transmitted through other modes. These have included donated organs and sexual transmission.
Epidemiology
Coccidioides (C immitis and C posadasii) is endemic in the soil in certain regions of the Western Hemisphere, almost all of which are located between latitudes 40° north and 40° south. [5, 17, 18] These areas are characterized by semiarid climates with hot summers and alkaline soil.
Although residents of endemic areas are at highest risk of acquiring infection, coccidioidomycosis increasingly is being recognized outside of these areas due to travel, population mobility, immunosuppression, and reactivation. Diagnosis often is delayed in nonendemic areas because coccidioidal infection initially is not considered in the differential diagnosis. [5, 6, 7, 8]
United States statistics
In the United States, C immitis is endemic in California's San Joaquin Valley and southeastern Washington, whereas C posadasii is endemic in areas of the desert Southwest, including southern parts of Arizona as well as certain parts of Utah, Nevada, New Mexico, and Texas. Coccidioidomycosis occurs both among residents of these endemic areas as well as travelers who visit there. An occasional case transmitted via fomites is reported outside of endemic areas.
Historically, people at greatest risk for contact include farmers, construction workers, and archaeologists. [19, 20] Thus, otherwise healthy persons exposed to high spore burdens have a higher likelihood of more severe disease.
The exact incidence of coccidioidal infections only can be inferred because approximately 60% of those infected are asymptomatic or have subclinical disease and never come to medical attention. An estimated 150,000 infections occur annually in the United States. This estimate is higher than the 100,000 cases per year previously cited in the literature and reflects population increases in southern Arizona and central California, where the organism is endemic. [10, 15] From 1988-2011, the incidence of coccidioidal infections in Arizona, California, Nevada, New Mexico, and Utah increased from 5.3 per 100,000 to 42.6 per 100,000. [21]
Approximately 25,000 new, clinically evident cases of coccidioidomycosis are reported annually in the United States. As many as 75 deaths per year result from the infection.
Based on skin test data, 80% or more of residents living in endemic areas for 5 years or longer will have a positive coccidioidin skin test result. The prevalence of positive skin test results ranges from 50-70% in the southwestern United States and increases with age.
Several sharp upsurges in the incidence have occurred. The western migration of the 1930s and the influx of military personnel in the 1940s triggered notable increases. In 1978, the first true epidemic occurred after an unprecedented dust storm that originated in the lower end of the San Joaquin Valley, quadrupling the incidence of disease.
A coccidioidal epidemic occurred in California in 1991-1994. In 1992, this outbreak produced a peak of approximately 4200 cases, an increase of more than 14-fold from baseline. One explanation for the epidemic is that it occurred after a 5-year drought that was terminated by above-average rainfall. This rainfall allowed dormant arthrospores to germinate and to be carried aloft by summer winds. At the same time, a marked influx of disease-naive individuals into the area further set the stage for the epidemic.
In areas of highest endemicity, the infection rate is approximately 2-4% per year. The prevalence in endemic areas has varied over time; the disease affects 30-40% of the population within the endemic regions of California and Arizona. [13] This figure is lower than findings from epidemiologic studies performed 50 years ago, when 68% of the population was found to have skin tests positive for coccidioidal antigens. Positive skin test results are related to the duration of residence in endemic areas and to occupational and recreational exposure to dust.
The number of cases of coccidioidomycosis in endemic regions rises sharply in the late summer and early fall, after the soil dries. At that time, soil disturbances, either natural (wind) or man-made (agricultural endeavors, construction, archaeological excavations), are likely to send Coccidioides spores airborne, enhancing the likelihood of its inhalation. In particular, outbreaks have been documented after earthquakes (eg, the 1994 Northridge earthquake in California) and wind storms.
Arizona, where coccidioidomycosis is a reportable condition, has the greatest number of cases. This likely represents symptomatic cases only. More than 5,000 cases are reported annually in Arizona, and the state has noted a steady increase in cases, with 7 cases per 100,000 persons in 1990, increasing to 15 cases per 100,000 persons in 1995 [22] and an estimated 75 cases per 100,000 persons in 2007.
In California, the number of annually reported coccidioidomycosis cases more than tripled from 2000-2006, rising from 2.4 to 8 cases per 100,000 population. [23] The annual incidence was highest in Kern County (150 cases per 100,000 population), with the hospitalization rate highest among non-Hispanic blacks, increasing from 3 cases to 7.5 cases per 100,000 population.
Coccidioidal disease has a significant socioeconomic impact in the United States. An otherwise healthy individual diagnosed with symptomatic coccidioidomycosis may miss more than one month of school or work. Recent estimates of antifungal medication costs range from $5000 to $20,000 per person per year of therapy for the disease. [6, 24]
Scholarship athletes represent a precisely defined group to calculate endemic risk for infection within a young adult population. [25] Case rates were higher for scholarship athletes in Tucson, Arizona, than for other students, highlighting the need to routinely test students for coccidioidomycosis in endemic areas.
International statistics
Coccidioidomycosis is a disease of the American continents. It first was described in Argentina in the late 1800s, and most of what is known today was due to investigations made in the United States in the early 1900s. All countries in between have described cases of coccidioidomycosis, and most have recognized endemic areas.
The incidence of coccidioidal infections in other endemic areas of North and South America outside of the United States is unknown. The infection risk remains highest in the endemic areas of northern Mexico and Central and South America. However, the incidence over time may not mirror the increase seen in the southwestern United States, because the latter increase has been driven by construction and immigration of uninfected individuals into the area.
The prevalence of coccidioidomycosis in the Americas is as follows [26] : 56% in Mexico, 42% in Guatemala, very low prevalence in Venezuela and Colombia, 26% in Brazil, 44% in Paraguay, and 40% in Argentina. In an endemic area of Brazil, coccidioidomycosis has been linked to hunting of armadillos. [18]
The incidence of coccidioidomycosis outside of the Western Hemisphere is extremely low, but cases (imported from California, Arizona, and Mexico) have been reported in the following countries:
Racial differences in incidence
No race predilection for primary infection with Coccidioides species has been observed. However, the risk of dissemination or progressive pulmonary disease is higher in Filipinos and blacks, and possibly in other Asians, Hispanics, and Native Americans. [33] The risk for dissemination is 175 times greater in Filipinos and 10 times greater in Blacks than in non-Hispanic Whites. [10] Hispanics with A or B type blood groups also have a slightly higher risk for advanced and/or disseminated disease compared with the population as a whole. [34]
This risk persists when analyses are controlled for age, sex, additional demographic features, concurrent medical problems, duration of exposure, and occupation. [35] When these populations are infected with the Coccidioides organism, their rate of skin test positivity decreases, and their complement-fixation titer increases compared with findings in the non-Hispanic White population.
One large study of 536 individuals demonstrated that 2.6% of non-Hispanic whites had dissemination, compared with 3.4% of Hispanic individuals, 7.3% of Filipinos, 22% of African Americans, and 20% of Asians. [36]
Sexual and age-related differences in incidence
An increased incidence of primary coccidioidal infection may be apparent in older boys and men because of occupational exposure. Women who are pregnant, especially during the third trimester and in the immediate postpartum period, are at higher risk for dissemination than the general population. [10, 37, 38] Recent evidence suggests these risks are biologic rather than sociologic as male dogs and primates also appear to be at increased risk. [39]
All age groups can be affected. Primary infection of the newborn rarely occurs. [38] Infection of the genital tract of the mother can result in placental involvement, coccidioidal endometritis, and aspiration of infected amniotic fluid by the fetus. Both congenital and perinatal transmission of Coccidioides species have been reported. However, infants can experience severe disease within the first few months of life, especially if exposed to a large respiratory inoculum.
Prognosis
Most patients with coccidioidomycosis have an excellent prognosis; most infections are self-limited and resolve within a few months without the need for medical intervention. In more than 90% of symptomatic individuals, no further sequelae develop. Treatment with antifungal therapy is effective in most of the defined clinical syndromes, however, and therefore the prognosis for recovery in these patients is also excellent.
Chronic coccidioidomycosis develops in 5-8% of patients following primary pulmonary disease. This is characterized by pulmonary disease, with or without extrapulmonary spread, or by extrapulmonary disease alone. Chronic pulmonary disease generally represents failure of local defenses and is commonly associated with advanced age and/or diabetes. The most common forms are cavity or nodule formation, which frequently represent a transition from acute disease to resolution.
Fewer than 1% of patients progress to disseminated disease. Patients with suppressed immune systems or those taking immunosuppressant medications are especially at risk for progressive or disseminated disease, and their coccidioidal infections may be difficult to eradicate.
Factors associated with increased risk for more severe disease include the following:
-
HIV disease, especially when CD4+ cell counts are < 250/mL
-
Pregnancy; the risk is slightly higher with each progressive trimester
-
Lymphoma
-
Solid organ transplantation
-
Long-term corticosteroid treatment
-
Treatment with tumor necrosis factor (TNF)-alpha inhibitors [40]
Disseminated disease may require months to years of antifungal therapy, and high-risk patients are at significant risk for relapse when treatment is stopped, even after extended courses (years) of treatment.
Coccidioidal IgG titers may be useful to assess risk for dissemination (titers >1:16) and monitor response to therapy (fourfold or greater decline in titer).
Although morbidity is substantial in coccidioidomycosis, mortality is very low; the mortality rate is approximately 0.07%. Death occurs most commonly in patients with disseminated disease, underlying risk factors, or immunosuppression. In immunocompromised patients, mortality can be as high as 70% even with appropriate therapy. [5, 6, 7] Few patients with advanced HIV infection who develop Coccidioides infection have survived longer than a few months.
In disseminated disease, the mortality rates in neonates and infants are much higher than those seen in children, adolescents, and adults. Septic shock may develop, especially in older or immunocompromised patients, and unfortunately, the outcome in these patients is uniformly poor.
Of the clinical syndromes, mortality is highest in coccidioidal meningitis. If left untreated, meningitis is fatal in 90% of patients within 1 year and is universally fatal within 2 years. Mortality rates can be 20-40%, even with treatment.
Mortality is significantly increased in coccidioidal meningitis patients with complications such as hydrocephalus or infectious arteritis. Hydrocephalus is the most common complication (30%) and carries a mortality rate of 40%. Occlusion of the cranial vessels by inflammatory exudates may lead to stroke. Occlusion occurs in 10% of patients and increases the likelihood of mortality. [41]
Patient Education
Residents of and travelers to endemic areas should be aware of the risk for coccidioidal infection. Education about the possibility of acquiring infection through exposure to dust or soil and the avoidance of avoiding activities that increase the likelihood of dust inhalation (eg, recreational activities, construction, archaeological digs) is particularly important for patients at high risk (eg, immunocompromised patients, pregnant women, African Americans, Filipinos, those with diabetes).
For patient education information, see the Procedures Center, as well as Bronchoscopy.
-
Soft tissue abscess due to cocci.
-
Pulmonary cocci spherule (Hematoxylin-eosin stain).
-
Pulmonary cocci spherule, periodic acid-Schiff stain.
-
Erythema nodosum can be observed in coccidioidomycosis, tuberculosis, histoplasmosis, drug reactions, and streptococcal infections.
-
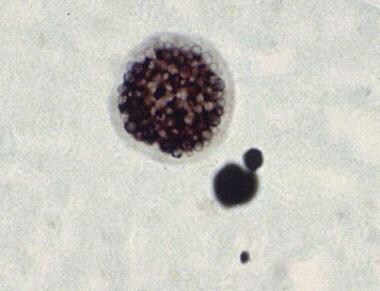
A Coccidioides immitis spherule containing endospores. Courtesy of Thomas Matthew.
-
Arthroconidia become airborne and infect the human host to begin the parasitic phase of its life cycle. The arthroconidia develop into spherules containing endospores, which propagate infection in human tissues. Courtesy of Thomas Matthew.
-
A granuloma with coccidioides immitis spherule (pretracheal lymph node biopsy).
-
A ruptured Coccidioides immitis spherule (pretracheal lymph node biopsy).
-
Gomori methenamine silver stain of Coccidioides immitis spherule (pretracheal lymph node biopsy).
-
Periodic acid-Schiff stain of Coccidioides immitis spherule (pretracheal lymph node biopsy).
-
Coccidioidal spherules rupturing and releasing endospores. Gomori methenamine silver (GMS) stain. Photograph by Joseph Rabban, MD.