Background
Minimal access surgery (MAS) has been in existence since the early 19th century. In the form of laparoscopy, MAS has been used by gynecologists since the 1960s. Its application to general surgery began when Muhe performed the first laparoscopic cholecystectomy in 1985. In 1987, Mouret and Dubois helped popularize this procedure, and laparoscopic cholecystectomy soon became the standard of care. [1] Since then, MAS has been applied to numerous other procedures both in the abdomen (laparoscopy) and in the chest (thoracoscopy), with good results.
The advantages of MAS were realized by surgeons operating on adults long before this approach was accepted in the pediatric community. Initially, performing MAS in the pediatric population was resisted for the following reasons:
-
It was widely believed that children did not experience pain as adults did
-
The cost of laparoscopy was believed to be too high
-
The available equipment was not small enough
-
MAS in children was regarded as too demanding to perform and too difficult to learn
-
The cases were thought to take too long to set up and to perform
-
Many surgeons believed that laparoscopic approaches did not really apply to children, and the need for cholecystectomy was relatively uncommon in children
-
Pediatric surgeons already prided themselves on their ability to work with small incisions
-
Many believed that MAS was not safe and that its efficacy was not proved
In 1973, Gans and Berci were the pioneers in pediatric laparoscopy. [2] They performed laparoscopy (ie, peritoneoscopy) on 16 children aged 1 day to 14 years, mainly for diagnostic purposes and for obtaining biopsy specimens. After that initial experience, however, there was a long lag period before pediatric surgeons in Europe and the United States picked up the torch in the early 1990s, after which point expertise in abdominal [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19] and thoracic operations [20, 21, 22, 23, 24, 25] started growing rapidly and began to be disseminated widely.
Poor-quality pediatric laparoscopic instruments and telescopes that were not small enough were perhaps the most significant barriers to the advancement of pediatric MAS.
In a comparative 5-year study, the outcomes of 211 children who underwent MAS were compared with age-matched controls with similar diagnoses who underwent open surgery. [26] No significant differences in mortality or morbidity were found. However, the hospital stay was shorter for children who underwent laparoscopic cholecystectomy, appendectomy, nephrectomy, splenectomy, and surgery for intra-abdominal testis than for those who underwent open surgery. In addition, all parents favored the cosmetic results of MAS.
Additional procedure-specific trials yielded equivocal results for laparoscopic appendectomy [27, 28, 29, 30] and demonstrated similar or superior results for laparoscopic urologic procedures, [31, 32] whereas laparoscopy was found to be advantageous in the correction of inguinal hernias [33, 34, 35] and hypertrophic pyloric stenosis. [36]
Subsequently, robotic-assisted laparoscopy and thoracoscopy have been adopted for use in children at numerous centers, though this transition has been constrained by the issues surrounding the adaptation of such approaches to smaller patient anatomies. [37, 38, 39]
Refinements and innovations in adult surgical practice have frequently been adapted for use in infants and children; however, many of the advances made in pediatric laparoscopy have also migrated back into adult laparoscopy. As the smaller telescopes and instruments developed for pediatric MAS became more broadly available, they were rapidly adopted by adult surgeons.
Physiologic Effects of Insufflation
Insufflation of the abdomen or chest cavities for MAS procedures has important physiologic effects, which have been extensively studied in adults and children. [40, 41, 42, 43]
Although suspension of the abdominal wall has been suggested as an alternative to pneumoperitoneum, [44] technical difficulties have precluded the broad adoption of this approach, [45, 46] and insufflation of the abdomen continues to be generally regarded as a prerequisite for successful laparoscopy.
Several alternative gaseous media were investigated for use in abdominal insufflation in the late 1990s and early 2000s, [47, 48, 49] but carbon dioxide gas remains the standard for MAS. [50, 51] Carbon dioxide is easily absorbed and eliminated through the lungs, and thus, the risk of clinically significant air embolism (gas bubbles entrapped within blood vessels) is lower than with other gases. In addition, carbon dioxide suppresses combustion, reducing the danger of fires in the operative field when electrocautery and other forms of energy devices are used.
Circulatory
Under normal conditions, intra-abdominal pressure varies widely, and it can increase transiently to levels as high as 200 mm Hg (eg, during forceful coughing and straining during defecation). Certain medical therapies also modestly increase intra-abdominal pressure (eg, 2-8 mm Hg for peritoneal dialysis), without adverse effects.
Insufflators deliver gas under pressure, and once the delivered gas volume exceeds the ability of the peritoneal cavity to expand, intra-abdominal pressure rises rapidly. If unchecked, the pressure could exceed pathologic limits, leading to detrimental physiologic effects. This is especially true when the cavity is small, as in children. The ability of the abdominal cavity to accommodate an increase in pressure depends on the pressure applied, the compliance of the abdominal wall, and the length of time during which the pressure is maintained.
Increased intra-abdominal pressure interferes with infradiaphragmatic venous and arterial blood flow, especially to the kidneys. The pressure at which this occurs is variable, depending on the size of the patient, the central venous pressure, and the mean arterial pressure. This pressure change can act to limit the return of blood to the heart from the lower limbs and abdomen.
While the arteries remain unobstructed, increased pressure on the abdominal aorta is transmitted to the heart, and cardiac output is decreased as the left ventricle pumps against this increased pressure gradient. This typically results in an increased heart rate compensating for a smaller stroke volume and a net increase in stroke work. In children with preexisting decreased cardiac output, increased intra-abdominal pressure may lead to acute cardiac failure. As a general rule, the insufflation pressure used for a procedure should be only as high as is necessary to provide adequate visualization.
Respiratory and metabolic
Insufflation may displace the diaphragm into the chest cavity, decreasing total lung capacity and functional residual capacity and adding to the acid-base disturbance. Insufflation pressures of 12 mm Hg increase peak airway pressure (PIP) by 40% and decrease lung compliance by 47%, with no change in dead space. Pulmonary arterial pressure and pulmonary wedge pressure both increase with pneumoperitoneum, improving ventilation-perfusion at intra-abdominal pressures of less than 12 mm Hg. This may help explain the lack of effect on partial pressure of oxygen under these conditions.
Increased minute ventilation (ie, increased rate, airway pressure, or tidal volume) can compensate for pulmonary mechanical restriction due to elevated intra-abdominal pressures. The carbon dioxide is mostly absorbed across the peritoneal surface, and a rise in its partial pressure can be offset by increasing minute ventilation.
Other
Increased intra-abdominal pressure can exacerbate gastroesophageal reflux (GER), adding to the perioperative risk of aspiration. Migration of insufflated gas out of the abdomen (as occurs when surgical dissection enters adjacent cavities, such as the chest) can cause patient discomfort or impair respiratory or cardiac function. Coronary, hepatic, mesenteric, and renal flow may be affected, as well as cerebrospinal fluid (CSF) pressure and pulmonary dynamics.
Measures to mitigate effects
Limiting exposure to insufflation while maintaining a visible surgical field is essential for safe MAS. The ventilatory and circulatory changes can be appreciated within minutes of the onset of insufflation of gas. Pressures in excess of 15 mm Hg are associated with significant pathophysiologic effects, but these are reversible over a 2-hour period. In infants and children, no hemodynamic effects are observed at a pressure of 10 mm Hg for less than 15 minutes.
The pressure delivered by modern insufflators is limited, typically not exceeding 15 mm Hg in adults. Pressure limits vary in infants and children, and care must be taken to start with low flow (eg, 1 L/min) and to maintain the lowest pressure required to obtain visualization (6-8 mm Hg for infants, 8-12 mm Hg for toddlers and school-age children, and 12-15 mm Hg for adolescents and older patients).
Ventilation and insufflation strategies should be promptly altered as required to maintain normal physiology (ventilatory mechanics and hemodynamic performance). In the event of abrupt or progressive decompensation, insufflation should be halted and pneumoperitoneum evacuated. To the extent possible, all insufflated gas should be evacuated upon conclusion of MAS.
Elevation in carbon dioxide may continue for several hours following MAS. Continued monitoring of the cardiac, respiratory, and renal systems should be carried out in the immediate postoperative period, particularly when opioid analgesics are used, because these may amplify respiratory depression.
Advantages
Advantages of MAS include the following:
-
MAS (laparoscopy and thoracosopy) often offers better visualization than open surgery—in particular, better visualization of the esophageal hiatus and deep structures in the pelvis
-
MAS offers dramatic advantages in terms of recovery and quality of life after the operation
-
Postoperative pain is reduced, which decreases postoperative narcotic use and its complications; this also aids in lowering the incidence of pulmonary complications
-
Smaller wounds are associated with fewer wound complications, less scarring, and better cosmesis
-
MAS results in reduction of postoperative adhesions
-
Patients stay in the hospital for a shorter period and recover faster
-
Patients are able to return more quickly to their normal activities (eg, feeding, school, and work)
-
A child's quick recovery allows parents to return to work faster
-
Video imaging allows surgical assistants, anesthesiologists, and nurses to view what the surgeon is doing and to actively participate in the procedure in their respective roles [52]
-
Older patients, particularly those requiring treatment for malignancy, benefit from fewer complications and shorter hospital stays [56]
Disadvantages
Disadvantages of MAS include the following:
-
Initial capital cost is associated with MAS because new equipment and training are necessary.
-
Operating time may be longer and the complication rate higher during the learning curve of the procedure.
-
Tactile sensation is decreased (or absent, in the case of robotic-assisted surgery), and adjustment to this decrease is most difficult when the surgeon is learning MAS techniques; experience diminishes this effect over time, and other methods of overcoming the effect are useful, including conscious awareness of visual cues and use of imaging modalities (eg, intraoperative ultrasonography [US] and fluoroscopy).
-
Because most video systems provide only a two-dimensional (2D) image, depth perception is affected, though experience diminishes this effect over time; three-dimensional (3D) video systems are available for use in MAS (including the robotic platforms), but the value of such systems is disputed, particularly by surgeons experienced in MAS
-
Control of bleeding was difficult when MAS was first adopted because of the limitation of energy sources to monopolar electrocautery; however, this problem has been addressed by the introduction of multiple energy devices using ultrasonic and bipolar technologies adapted for use in MAS; nonetheless, bleeding remains one of the most common reasons for conversion to open surgery
-
The number of instruments that can be used and the angles at which they can be applied are limited; training courses can help surgeons learn to use these instruments to better effect for both dissection and tissue handling, as well as intracorporeal suturing; robotic systems using wrist technology may address this problem as well, but they currently require the use of larger instruments
Complications
Technique-related
Complications can occur with placement of the initial trocar or the initial creation of pneumoperitoneum, including injury to the underlying vessels or viscera. These injuries can be minimized by the use of open technique for the first trocar placement. [52, 57] Complications occuring as a result of insufflation include air embolus and arrhythmia—both of which can lead to sudden cardiovascular collapse. The chances that either of these will occur can be reduced by confirming intra-abdominal placement of the initial port before insufflation and slow flow of carbon dioxide.
Complications can also arise from dissection during the procedure. These include direct injuries to hollow and solid organs, as well as thermal injury from energy devices. These can be minimized by optimizing visualization and employing careful and precise technique.
Carbon dioxide–related
Carbon dioxide can easily be absorbed through the peritoneal surface, leading to hypercapnia. Elevation in carbon dioxide can lead to acidosis, which can have further metabolic and hemodynamic consequences. Insufflation of carbon dioxide can cause cardiovascular compromise because of the previously mentioned decreased venous return. Hypothermia can also ensue because of cold carbon dioxide insufflation, especially in small infants.
As mentioned above, another serious but fortunately rare complication is gas embolism; this is minimized by using carbon dioxide instead of other gases. This complication has also been reported during insufflation in infants when an umbilical incision is used for entry. Inadvertent preperitoneal insufflation in a patient with a patent umbilical vein can lead to devastating consequences [58] and should be considered in choosing access techniques for patients younger than 1 month.
These complications can be minimized with the use of low pressure, slow flow, and warm humidified gas insufflation. In addition, slight hyperventilation, proper fluid resuscitation, and careful monitoring in the operating room (OR) will also protect the patient from inadvertent negative effects of pneumoperitoneum.
Applications in Foregut
Esophageal surgery
Operations on the esophagus with standard open approaches are associated with significant morbidity that is directly related to the operative incisions, as follows:
-
Thoracotomies are associated with significant postoperative pain and compromised pulmonary toilet; in addition, in children, long-term morbidities may include chest-wall asymmetry, muscular atrophy, scoliosis, and even breast maldevelopment; the younger the patient at the time of surgery, the higher the associated risk of these complications
-
Upper abdominal incisions have a significant effect on pulmonary toilet and are associated with an increased oxygen requirement and an increased risk of postoperative atelectasis and pneumonia
MAS techniques offer alternatives to these morbid procedures.
Thoracoscopic repair of esophageal atresia and tracheoesophageal fistula was initially reported reported by Rothenberg and Lobe in 1999. [59] Fine dissection and elegant suturing of the esophageal anastomosis require significant technical skills. Robotic surgery may play a role in this procedure in the future, but at present, applications are limited by the size of the robotic instruments.
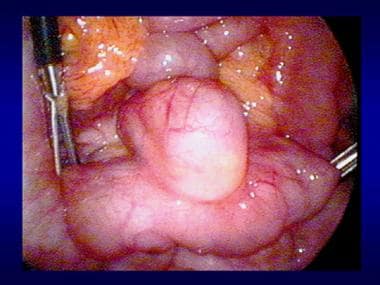
Esophagomyotomy (Heller myotomy) with or without a partial fundoplication has successfully been performed with an MAS approach (including robotic-assisted procedures). A laparoscopic approach is ideal for patients with achalasia in that it allows adequate exposure for performing an anterior myotomy starting at least 4 cm above the esophagogastric junction (EGJ) and extending onto the stomach for at least 2 cm. Exposure of the thoracic esophagus through the hiatus is specifically advantageous over the open approach. A partial fundoplication (most commonly a Dor fundoplication) is performed, suturing an anterior fundic patch to both edges of the myotomized extramucosal incision. Upper endoscopy is simultaneously performed to ensure an adequate myotomy and evaluate for mucosal perforation.
Heller myotomy can also be performed via a thoracoscopic approach. (See the image below.)
Gastrostomy tube placement
Enteral access with a gastrostomy feeding tube is necessary in many children as a consequence of inability to swallow safely without aspiration or take in adequate calories. The majority of patients have neurologic impairment, but children with cystic fibrosis, malignancies, neurometabolic diseases, renal insufficiency, or congenital heart disease may also require enteral access for nutritional support.
Multiple MAS techniques have been described for gastrostomy tube placement. The advantages of a laparoscopic approach over a percutaneously placed gastrostomy include the following:
-
Precise placement of the gastrostomy in ideal locations on both the abdominal wall and the anterior wall of the stomach
-
Avoidance of injury to surrounding structures, including the colon and liver
-
Suture fixation of the stomach to the fascia
-
Placement of a primary low-profile gastrostomy tube
Operating time averages 30 ± 15 minutes. MAS-assisted percutaneous endoscopic gastrostomy (PEG) tube placement assures proper location of the tube in the stomach while avoiding injury to surrounding structures (particularly the transverse colon). It may also allow some flexibility in the site chosen for the tube.
Nissen fundoplication
The indications for and the technique of open fundoplication are well known to adult and pediatric surgeons. However, laparoscopic fundoplication offers excellent visualization of the hiatus, and after the initial learning curve, it can be expeditiously performed.
The morbidity of the surgical procedure (particularly with respect to respiratory complications, return of feeding, and length of hospital stay) is reduced with the laparoscopic approach. Significant respiratory advantages of an MAS approach to this procedure are recognized, particularly in pediatric patients with developmental delay. The likelihood of extubation after the procedure, the time spent in the recovery room, the time spent in the intensive care unit (ICU), and the time spent intubated are all reduced with MAS.
The laparoscopic technique is similar to the open technique and is typically performed with five ports, including the camera port. An angled (30° or 45°) scope is used. This procedure can also be combined with a gastrostomy feeding tube if necessary.
A previously placed gastrostomy tube can limit visibility and necessitate modification of the port placement or even revision of the gastrostomy site. In patients with contractures, care must be taken to position, pad, and secure them on the OR table in order to overcome the technical challenges. (See the video and images below.)
Pyloromyotomy
Laparoscopic pyloromyotomy is typically performed with three incisions: a camera port at the umbilicus and two stab incisions, one in each upper quadrant. [1] The average operating time is approximately 15 minutes. Most patients (>90%) are discharged home within 24 hours.
Although the laparoscopic approach is not universally accepted, the Accreditation Council for Graduate Medical Education (ACGME) National Resident Report for 2018-2019 showed that more than 90% of pyloromyotomies done by pediatric surgery trainees were done laparoscopically. This is likely to translate into wide acceptance in the coming years.
Open techniques, including the Ramstedt pyloromyotomy (right-upper-quadrant [RUQ] incision) and umbilical approach, are still performed in some centers. There is some evidence to suggest that the complication rate is lower with the laparoscopic approach. In addition, the cosmetic results of laparoscopic pyloromyotomy are superior to those of the open procedure performed via the traditional RUQ incisions. (See the videos and images below.)
Repair of duodenal atresia or stenosis
Congenital duodenal obstruction is a congenital anomaly in which the first portion of the small bowel, the duodenum, is completely or partially obstructed. Causes include duodenal atresia, duodenal stenosis or web, and annular pancreas. Many infants with this anomaly are diagnosed antenatally on the basis of a large stomach, a “double-bubble” sign on US, and polyhydramnios (excessive amniotic fluid) due to obstruction of the proximal gastrointestinal (GI) tract.
With the use of three 3- to 4-mm ports, the obstruction can be bypassed with a duodenoduodenostomy connecting the duodenum proximal to the obstruction to the duodenum beyond the obstruction. Care must be taken to avoid injury to the ampulla of Vater and to make an adequate-sized anastomosis.
Initial reports raised concerns about a possibly higher complication rate. [60] However, subsequent evidence showed no difference between open and laparoscopic approaches. [61] In addition, MAS may have advantages in terms of postoperative morbidity, time to full feedings, and hospital length of stay. [62, 63] The main barriers to acceptance of this technique continue to be (1) surgeon comfort with MAS in infants and (2) access to appropriate equipment.
Cholecystectomy
The first pediatric laparoscopic cholecystectomy was reported by Sigman et al in 1991. [64] Although laparoscopic cholecystectomy is one of the most common laparoscopic procedures in adult patients, it is less common in pediatric patients because of the lower incidence of gallstones in children. Nevertheless, the number of pediatric cholecystectomies performed is increasing each year, and more than 95% are done laparoscopically in pediatric surgery training programs, according to the ACGME National Resident Report.
Many of the pediatric patients who require laparoscopic cholecystectomy have blood dyscrasias and form pigment stones. Biliary dyskinesia, a condition characterized by an abnormally low ejection fraction of the gallbladder, is less common than symptomatic biliary colic but is certainly encountered by pediatric surgeons. Although surgical indications are not universally accepted, in select patients, laparoscopic cholecystectomy may alleviate symptoms.
Like its adult counterpart, pediatric laparoscopic cholecystectomy is performed with four ports, including the camera port. The size of these ports ranges from 2 to 10 mm. There is also a single-incision option that can be performed either by means of standard laparoscopy or with robotic assistance. [65]
Laparoscopic cholecystectomy has been shown to be safe, even in an infant (< 19 months). [66]
Applications in Midgut
Small-bowel surgery
Like a feeding gastrostomy tube, a jejunostomy tube can be placed laparoscopically.
Small bowel can be resected if necessary, with an intra- or extra-abdominal anastomosis, by means of an MAS technique.
Laparoscopy has been used to treat intestinal malrotation, [55, 67] intussusception, [68] adhesiolysis, [55] Meckel diverticulum, [69] and small-bowel atresia, as well as enteric duplication cysts. (See the image below.)
 Laparoscopy in a patient with gastrointestinal bleeding. Laparoscopic view of the Meckel diverticulum.
Laparoscopy in a patient with gastrointestinal bleeding. Laparoscopic view of the Meckel diverticulum.
Appendectomy
Appendectomy is the most frequently performed abdominal procedure in children. Laparoscopic appendectomy was developed in the early 1980s by the German gynecologists Semm and Schrieber. [70, 71] In 1991, Valla et al reported the first series of pediatric laparoscopic appendectomies. [71]
Laparoscopic appendectomy has become standard in pediatric medical centers. According to the ACGME National Resident Report, fewer than 2% of appendectomies by pediatric surgery trainees were done via an open approach. The laparoscopic approach offers many of the same advantages for appendectomy that were outlined previously for other operations. (See the video below.)
Early studies suggested no major differences between open and laparoscopic appendectomy techniques. [52, 72, 27, 30] Other trials and cohort studies comparing laparoscopic and open appendectomy demonstrated advantages with MAS, including decreased postoperative pain, improved cosmesis, and shorter times to ambulation and discharge, though some benefits were confined to children with uncomplicated apppendicitis. [28, 29]
Laparoscopy is even more useful if the diagnosis of appendicitis is in question. It affords better visualization of the rest of the abdominal cavity and pelvis, allows better irrigation of the peritoneal cavity, and yields a lower wound infection rate.
Three ports (2-5 mm) are typically used, though single-port appendectomy has also been described in the literature. [73]
Laparoscopy in children with chronic abdominal pain may provide valuable information as to the etiology of the pain. In cases where a source can be identified—for example, an ovarian cyst, a congenital band, or evidence of chronic inflammation of the appendix—a cure may even be possible.
Applications in Hindgut
Colon surgery
Laparoscopy has been used to treat diseases of the entire colon. [74] It also takes advantage of the excellent collateral blood supply of the colon, which makes mobilizing large segments possible.
As mentioned above, laparoscopy offers superb visualization of the pelvic structures, which makes working in the deep pelvis easier and safer. Once the colon is mobilized, it can be resected with an intra- or extra-abdominal anastomosis. The specimen can also be removed via the anus, and anastomosis can be performed transanally from the outside.
Classically, Hirschsprung disease has been treated by means of staged procedures involving biopsy, colostomy, pull-through, and colostomy takedown over a period of 6-12 months. However, with the help of MAS and extended transanal dissection, it can be performed as a single-stage procedure in most patients.
In 1994, Curran et al performed the first laparoscopic pull-through in a canine. [75] The procedure was then performed in humans in 1994 by Smith et al. [76] Laparoscopic pull-through has been shown to be feasible in infants younger than 3 months, with outcomes comparable to those of operations performed in older children. [77]
The procedure is carried out with three ports (3.5-5 mm) positioned to allow access to the sigmoid colon and pelvis; an additional port may be helpful if more proximal biopsies are needed. Although seromuscular biopsies have been described, full-thickness biopsies provide a more accurate pathologic evaluation in looking for ganglion cells and hypertrophic nerves. Once the level of normal ganglion cells and nerves is identified, the blood supply of the distal colon (inferior mesenteric artery [IMA]) is ligated. The rectum is then mobilized circumferentially down to the levator musculature in girls and the prostate in boys. (See the video below.)
The dissection is then begun transanally, and colon is removed to the level of biopsy-proven ganglionic cells. There are three different surgical approaches for Hirschsprung disease (Swenson, Soave, and Duhamel), and all of them can benefit from MAS assistance. Once the normal colon is identified and the Hirschsprung segment is resected, the anastomosis between the anus and neorectum is performed above the dentate line. [52]
A case series by Li et al reported a mean operating time of 160 minutes, a mean time to return of bowel function of 22 hours, and a mean length of postoperative hospital stay of 8 days. [78] A meta-analysis by Tomuschat et al reported postoperative morbidities of 9.4% for enterocolitis, 6.5% for incontinence, 11.1% for constipation, and 5.8% for reoperation. [79]
For those patients with transition zones proximal to the splenic flexure, an additional port may be helpful in obtaining mapping biopsies through the remaining colon. If long-segment disease is suspected, consideration of an ileostomy and planning for a staged procedure when the child is a little older are recommended. This allows the final pathology to be available and the growth of the colonic arterial arcade to mature, both of which enable all options for reconstruction to be considered.
Repair of anorectal malformations
Most surgeons repair anorectal malformations (ARMs) in patients with a perineal fistula primarily in the newborn period without a colostomy. For all other anorectal defects, many still defer to the three-step approach, which consists of (1) diverting colostomy shortly after birth, (2) repair of the ARM at a later date, and finally, (3) colostomy closure. [80] Currently, there is growing interest in repairing ARMs earlier in life. In select patients, there is an increasing trend toward performing primary procedures for higher ARMs without a protective colostomy. [80]
The laparoscopic-assisted anorectal pull-through (LAARP) is even more attractive because it allows repair of the defect without laparotomy and, in some cases, without colostomy. [81] This procedure has particular applicability to ARM patients with a bladder neck fistula or higher. The rectum can be mobilized off of the bladder via a laparoscopic approach, with care taken to avoid injury to the bladder and to divide the fistula so as not to leave a remnant. A 2019 study that reviewed all symptomatic ARM patients presenting to a tertiary referral center demonstrated that about 9% had a remnant of the original fistula (ROOF).
Reports of long-term results following LAARP are sparse, but the literature suggests a lower risk of perineal wound dehiscence, a nonsignificantly increased risk of anal stenosis, and a possible increased risk of rectal mucosal prolapse. Functional outcomes following LAARP, particularly regarding voluntary bowel movements, incidence of soiling, and constipation, have not been shown to differ from those of open surgery, but the type and severity of malformation are significant confounding factors, and further study is required. [82]
Applications in Abdominal Wall, Scrotum, Testes, and Ovaries
Herniorrhaphy
Conventionally, hernia surgery in children is performed via high ligation of the hernia sac. This requires incision over the inguinal canal and dissection to expose the cord structures and hernia sac. In all but infants in whom the internal and external rings overlap, this requires extending the incision through the abdominal wall and opening the inguinal canal. The hernia sac is then isolated from the cord structures, a high ligation of the sac is performed, and the incision is closed in layers, with the inguinal canal reapproximated when necessary.
The contralateral side is commonly evaluated laparoscopically during an open inguinal hernia repair by inserting the port through the hernia sac, inflating the abdominal cavity, and inspecting the contralateral side with a 70º laparoscope. There is some debate about whether a patent processus vaginalis will become a clinical hernia and warrants repair. This finding is present in as many as 38% of patients presenting for unilateral hernia repair, [83] though the overall incidence of contralateral hernia following unilateral repair is 6%. [84]
Criteria that have been proposed as indications to proceed with contralateral repair include the following:
-
Inflation of the contralateral hemiscrotum
-
Inability to see the end of the patent processus
-
Presence of bubbles with compression over the inguinal canal
Opponents of repairing the contralateral side quote the risk to cord structures and the inability to predict the development of a hernia.
Laparoscopic repair has also been reported via a transabdominal approach. A port is placed at the umbilicus, and the peritoneal cavity is insufflated with carbon dioxide. Various techniques have been described, most of which involve using a needle or other instrument to loop around the hernia sac (peritoneum) at the level of the internal ring. This performs a high ligation of the sac without a groin incision or dissection through the abdominal wall.
The ligation should be performed with nonabsorbable suture material—preferably a braided suture, given that monofilament suture repair is suture-dependent [85] and is associated with palpable knots. Cauterization of the peritoneum, avoiding the cord structures, has been described as inducing inflammation and promoting closure of the ring. [86]
The advantages of this technique include visualization of both sides and clear identification of indirect as well as direct inguinal hernias. In female children, there is very little risk to this approach; in male children, care must be taken to not entrap the cord structures in the repair. Hydrodissection, performed by injecting saline to separate the cord structures from the peritoneum, has been used to facilitate safe ligation of the sac. This type of repair does not address a concurrent distal (encysted) hydrocele.
MAS also has a theoretical advantage with respect to gonadal injury. In girls, the ovaries can be clearly visualized and incarceration identified. In boys, minimal or no dissection of the cord structures (including the vas deferens and gonadal vessels) occurs; therefore, the likelihood of injury to the testis is theoretically lower than with an open approach.
As with adult laparoscopic hernia repair, this approach has a practical advantage when repair of a recurrent hernia is undertaken. Because the surgical planes of the open approach are not violated, open repair of a recurrence should be less difficult. By contrast, reoperation following open repair requires dissection through scar tissue with the accompanying risk of injury to the cord structures.
In a systematic review of the published literature in English between 2009 and 2015, the International Pediatric Endosurgery Group reported that MAS approaches to the treatment of inguinal hernias in children resulted in lower postoperative complication rates and shorter operating times. [87]
Varicocele surgery
This procedure is usually performed with one to three trocars. [88] In one study, the median operating time was approximately 30 minutes, and the median hospital stay was 24 hours. Recurrence with the laparoscopic Palomo technique was low, and the rate of postoperative hydrocele formation was significant (6.6%). [89]
Identification and treatment of nonpalpable testes
MAS is indicated if the cryptorchid testis is not palpable in the inguinal canal after induction of anesthesia. The first report of abdominal testes identified by means of laparoscopy was in 1976, [90] and since then, laparoscopy has become the criterion standard for nonpalpable testes. Laparoscopy allows localization of intra-abdominal testes, detection of an absent testis, and identification of a canalicular testis. This localization is easily accomplished by following the course of the vas and the testicular vessels.
Once located, intra-abdominal testes can be treated by means of MAS-assisted orchidopexy. The nonpalpable testes are usually found between the internal ring and the external iliac vessels. [90] Once identified, the testicle is mobilized by taking down any gubernacular attachments and then dividing peritoneal attachments of the vas and vessels, with care taken to avoid injury to these structures. Adequate mobilization is usually achieved when the testicle is able to reach the contralateral internal ring. Placement of the testicle into the hemiscrotum can be achieved with different methods but most commonly involves passage along a new path just lateral to the ipsilateral pubic tubercle.
Management of ovarian pathology
Laparoscopy has been successfully used in a wide variety of gynecologic settings, including tubal torsion, adnexal torsion, and oophorectomy. [91] It is worth noting that the North American Society for Pediatric and Adolescent Gynecology (NASPAG) currently recommends ovarian-sparing surgery for cases of simple cysts, torsion (even if the ovary does not appear viable), or benign tumors.
Adaptation of an algorithm for the management of ovarian masses (including cysts) can prevent unnecessary oophorectomy without undertriaging rare malignant lesions. Imaging and biochemical markers, along with physical examination, can reliably predict malignancy; oophorectomy should be undertaken only when malignancy is suspected on the basis of this information. In cases where torsion is present and operative intervention is required before laboratory values and definitive imaging are available, detorsion without resection should be considered, with plans made for reoperation once data are available to inform the decision.
In teenaged girls with abdominal pain, diagnostic laparoscopy is invaluable. It can be performed easily via two or three ports and provides an excellent operative view. Early diagnosis of ovarian pathology (eg, torsion) and confirmation of disease processes (eg, endometriosis) can facilitate long-term management that preserves fertility. Patients do extremely well and are able to return to their preoperative activity relatively quickly.
Applications in Solid Organs
Splenectomy
In 1990, the MAS approach to splenectomy was first successfully performed in animals. [92] Since then, many accounts of laparoscopic splenectomy in humans have been reported. This procedure has now become the criterion standard for removal of the spleen. With the exception of malignancy, indications for laparoscopic splenectomy are the same as for the corresponding open procedure.
Patients are usually placed in a supine position with a bump under the costovertebral angle or in a 45° right lateral decubitus position. Typically, three to five ports of varying sizes (5-15 mm) are used. The larger port allows a large endoscopic bag to be used. Placement of this at the umbilicus yields an excellent cosmetic result. Compared with the open approach, the laparoscopic approach offers a much better view, without an extensive incision. Most of the dissection has been facilitated by the development of better energy modalities and improved stapling devices.
The spleen is placed in a bag, which is exteriorized and removed after the specimen is broken up with a finger or sponge forceps. Patients do extremely well postoperatively, and most are able to return home within 48 hours.
The MAS approach has been safely adapted to a variety of splenic procedures (including total and complete splenectomy, cystectomy, and splenopexy for wandering spleen), with low complication rates for well-selected patients. [93, 94, 95] (See the video below.)
Nephrectomy
Laparoscopic nephrectomy was first described in 1991 in adults, [96] and both transperitoneal and retroperitoneal approaches have been used. (See the video below.)
Ehrlich et al reported the first series of laparoscopic renal surgery in children. [97] He performed a total of 17 procedures, consisting of 10 nephrectomies, four nephroureterectomies, two partial nephrectomies, and one giant renal cyst excision. A transperitoneal approach was used in 17 children (age range, 4 months to 11 years), with good results. Ehrlich et al also reported the first laparoscopic partial nephrectomy in 1993.
Till et al performed a systematic literature-based search from 2011 to 2016 and concluded that conventional laparoscopy was still preferred over single-site or robotic-assisted methods and that these latter techniques required further study. [98]
The retroperitoneal approach [99] completely avoids the peritoneum, thereby decreasing the incidence of related complications (eg, postoperative ileus and postoperative adhesions). It is also ideal in patients who have had previous abdominal surgical procedures. This approach can be used for renal biopsy, nephrectomy, heminephrectomy, nephroureterectomies, nephropexy, adrenalectomy, and pyeloplasty. [100] The retroperitoneum is dissected by using a balloon, saline, a finger, or direct vision. Angled scopes provide a much better view because of the limited operational space. This approach has limitations in small children.
Laparoscopic donor nephrectomy (see the video below) has improved the operation for the donor and has increased the use of living related renal transplantation.
Other urologic procedures have also been performed laparoscopically, including pyeloplasty, bladder reconstruction, and ureteral reimplantation. [101, 102]
Adrenalectomy
The MAS approach can be applied to adrenal tumors, such as pheochromocytoma, and incidentally found adrenal masses. The MAS approach can be transperitoneal or retroperitoneal. MAS offers an excellent view of the surgical anatomy and the vasculature. The approach is similar to that followed in laparoscopic nephrectomy and has similar benefits.
Applications in Thorax
Thoracoscopy
Thoracoscopy, first introduced by Jacobeus in 1910 for dissection of tuberculosis (TB) adhesions, is safe and effective, even in infants weighing less than 1.5 kg, without significant morbidity or mortality. [53, 54]
As in the open technique, patients are placed in a lateral position with the operative side up. Three trocars are used in most cases, but additional ports to aid in retraction or exposure can be helpful.
Thoracoscopy is useful for the following [103] :
-
Repair of esophageal atresia/tracheoesophageal fistula
-
Aortopexy
-
Closure of patent ductus arteriosus [104]
-
Diaphragm plication [76]
-
Repair of congenital diaphragmatic hernia
-
Resection of subpleural blebs
-
Pleurodesis and pleurectomy
-
Pericardial drainage
-
Drainage of empyema
-
Lung biopsies
-
Tumor biopsies and resection of pulmonary metastases
-
Assessment or resection of mediastinal or lung masses [54]
-
Laparoscopic appendectomy.
-
Hirschsprung disease. Laparoscopic mobilization of the rectosigmoid.
-
Laparoscopic Nissen fundoplication.
-
Laparoscopic donor nephrectomy.
-
Laparoscopic pyloromyotomy.
-
Laparoscopic pyloromyotomy.
-
Splenic cyst.
-
Nephrectomy for multicystic kidney.
-
Types of minimal access surgery (MAS).
-
Historical resistance to pediatric minimal access surgery (MAS).
-
Pediatric miniature access surgery advantages.
-
"Ship in a bottle" analogy.
-
Laparoscopic appendectomy. Stapling the mesoappendix.
-
Laparoscopic appendectomy.
-
Laparoscopic appendectomy, identifying the mesoappendix.
-
Laparoscopic appendectomy. Stapling the appendiceal base.
-
Incision for an open splenectomy.
-
Hirschsprung disease. Laparoscopic mobilization of the rectosigmoid.
-
Splenectomy. Laparoscopic view of two accessory spleens.
-
Splenectomy. Stapling the splenic hilum.
-
Splenic cyst.
-
Laparoscopic cholecystectomy. Mobilizing the gallbladder.
-
Laparoscopic cholecystectomy. Transecting the cystic duct.
-
Hirschsprung disease. Barium enema showing the transition zone in the rectosigmoid.
-
Hirschsprung disease. Pull-through of aganglionic bowel and anoplasty.
-
Hirschsprung disease. Transanal approach. Mucosectomy and view of the circular muscle fibers.
-
Pectus excavatum.
-
Pectus excavatum.
-
Pectus excavatum.
-
Pectus excavatum.
-
Pectus excavatum.
-
Nephrectomy for polycystic disease.
-
Laparoscopic donor nephrectomy. Transecting the renal vein.
-
Thoracoscopy.
-
Laparoscopic Heller myotomy.
-
Esophagogram of a patient with achalasia.
-
Operations on the ovary. Ovarian cyst in a 10-month-old girl. The uterus and tubes are visible in the pelvis.
-
Operations on the ovary. Ultrasound of an ovarian cyst in a 10-month-old girl.
-
Operations on the ovary. The right tube is incomplete, probably an in utero event.
-
Operations on the ovary. Aspirating the ovarian cyst prior to removal.
-
Laparoscopy in a patient with gastrointestinal bleeding. Laparoscopic view of the Meckel diverticulum.
-
Laparoscopy in a patient with gastrointestinal bleeding. A positive Meckel scan.
-
Laparoscopy in a patient with gastrointestinal bleeding. A Meckel diverticulum.
-
Laparoscopy in a patient with gastrointestinal bleeding. The Meckel diverticulum and the adjacent ulcer are observed in the cut specimen.
-
Laparoscopy in a patient with gastrointestinal bleeding. Laparoscopy and extracorporeal bowel resection were performed through an umbilical incision and using two additional ports.
-
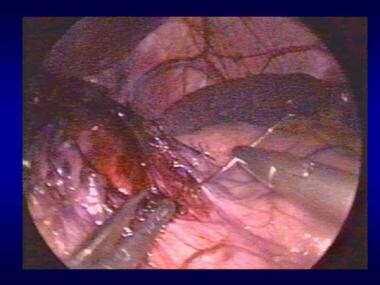
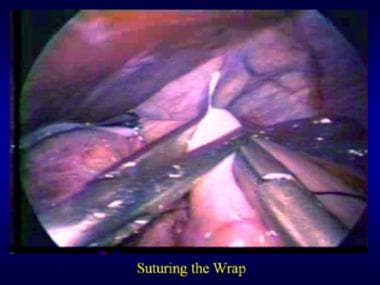
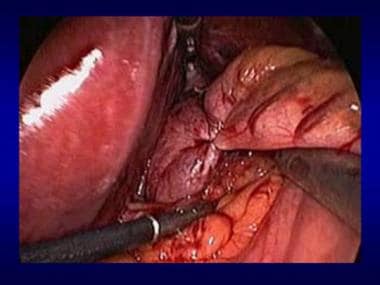
Nissen fundoplication.
-
Nissen fundoplication.
-
Open pyloromyotomy.
-
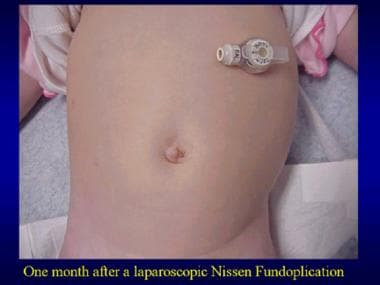
Nissen fundoplication. Postoperative view, with gastrostomy in place.
-
Pyloromyotomy. The pyloric "olive."
-
Laparoscopic pyloromyotomy.
-
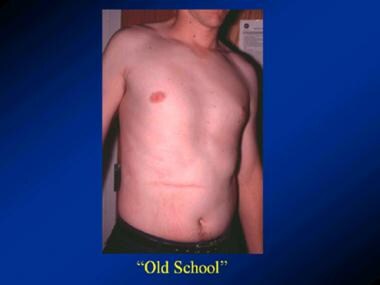
Pyloromyotomy. Scar in an adult who underwent open pyloromyotomy as an infant.
-
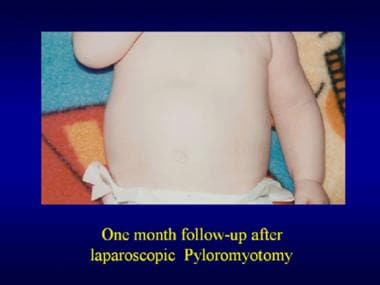
Pyloromyotomy. One month postoperatively after a laparoscopic pyloromyotomy.
Tables
What would you like to print?
- Background
- Physiologic Effects of Insufflation
- Advantages
- Disadvantages
- Complications
- Applications in Foregut
- Applications in Midgut
- Applications in Hindgut
- Applications in Abdominal Wall, Scrotum, Testes, and Ovaries
- Applications in Solid Organs
- Applications in Thorax
- Show All
- Media Gallery
- References