Practice Essentials
Pyloric stenosis involves narrowing and obstruction of the pyloric channel as a consequence of hypertrophy of the circular muscle of the pylorus. [1] It is the most common pediatric surgical disorder of infancy that necessitates surgery for associated emesis.
Autopsy findings of pyloric stenosis were first reported by Blair in 1717, but it was not until 1887, when Hirschsprung presented unequivocal clinical and autopsy findings of pyloric stenosis in two infants, that this entity became accepted. Adequate fluid resuscitation followed by pyloromyotomy is the standard curative treatment for pyloric stenosis.
Before 1912, early successful operative treatments of pyloric stenosis included gastroenterostomy, pyloroplasty, and forcible dilatation via gastrostomy (Loreta operation). In 1912, Ramstedt observed an uneventful recovery in a patient following pyloroplasty, in which sutures that were used in reapproximating the seromuscular layer had been disrupted. Following this observation, Ramstedt left the split muscle layer unsutured in all subsequent repairs.
The Ramstedt pyloromyotomy, whether performed through a right-upper-quadrant (RUQ) incision, through an umbilical incision, or via laparoscopy, [2] remains the standard operation for pyloric stenosis today (see Treatment).
For more information, see Pediatric Pyloric Stenosis, Pediatric Hypertrophic Pyloric Stenosis, and Imaging in Hypertrophic Pyloric Stenosis.
Anatomy
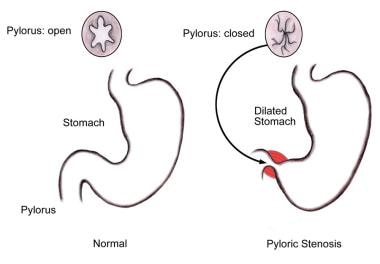
Pyloric stenosis involves hypertrophy of the circular muscle of the pylorus, resulting in narrowing and obstruction of the pyloric channel by compression of longitudinal folds of mucosa. Gastric distention results (see the image below). Gastric outlet obstruction results in emesis, which is characteristically nonbilious and projectile. Protracted emesis, as well as failure of the stomach to empty into the duodenum, results in progressive dehydration, electrolyte abnormalities, acid-base disorders, weight loss, and, potentially, shock.
Intraoperatively, the surgeon must pay strict attention to the serosal demarcation between the duodenum and the pylorus. The prepyloric vein, or Mayo vein, is located at this junction. The risk of duodenal perforation is prevented by stopping the distal extent of the myotomy 1-2 mm short of this point.
Pathophysiology
Pyloric stenosis involves hypertrophy of the circular muscle of the pylorus, resulting in narrowing and obstruction of the pyloric channel by compression of longitudinal folds of mucosa. Grossly, the pylorus is enlarged, resembling a tumor approximating the size and shape of an olive (ie, 2 cm long and 1 cm in diameter). Microscopically, the circular muscle hypertrophies, with increased connective tissue in the septa between the muscle bundles. An increase of chondroitin sulfate within the extracellular matrix may account for the cartilaginous quality of the pyloric tumor.
Gastric fluid loss is associated with the loss of H+ and Cl–. This fluid loss is unlike that in conditions caused by vomiting with an open pylorus, which involves losses of gastric, pancreatic, biliary, and intestinal fluid. Hypochloremic hypokalemic metabolic alkalosis is the characteristic biochemical disturbance observed in pyloric stenosis. Urinary Na+ and HCO3– losses, which compensate for Cl– losses, perpetuate this alkalosis.
With protracted vomiting, an extracellular volume deficit ensues, and urinary excretion of K+ and H+ increases in an attempt to preserve Na+ and volume. The initially alkalotic urine then becomes acidotic (paradoxic aciduria). This sign of protracted dehydration should alert the clinician to the severity of the volume and total body K+ deficit. The severity of electrolyte abnormalities depends on the duration of vomiting before resuscitation.
Greater awareness of the presenting signs of pyloric stenosis by pediatricians and primary care physicians, along with examination by means of ultrasonography (US), has resulted in earlier diagnosis and less severe electrolyte and acid-base abnormalities.
Etiology
No conclusive evidence for the etiology of pyloric stenosis exists; however, both hereditary and environmental influences are believed to be contributing factors. Multiple factors, including both neural and hormonal, have been implicated but not substantiated in the development of pyloric stenosis. An association with B and O blood groups and maternal stress during the third trimester has also been suggested. Although pyloric stenosis is now believed to be acquired, cases of pyloric stenosis diagnosed antenatally and in neonates have been reported.
Since 1976, several reports and cohort retrospective studies have appeared in the literature suggesting an association between pyloric stenosis and exposure to macrolide antibiotics (erythromycin). In 2002, Cooper et al suggested that early exposure to erythromycin (at 3-13 days of life) is associated with a nearly eightfold increased risk of pyloric stenosis (adjusted incident-rate ratio, 7.88). [3] No increased risk of pyloric stenosis was observed in infants exposed to erythromycin after 13 days of life.
In 1993, Huang et al, by homologous recombination, generated mutant mice (knockout mice) lacking the neuronal nitric oxide (NO) synthase (NOS) gene. [4] NO mediates nonadrenergic noncholinergic smooth-muscle relaxation throughout the gut. The stomachs of homozygous mutant mice were larger than normal in this group, and the circular muscle layer of the stomach and pylorus was hypertrophied. Wild-type mouse stomachs contained NOS in the myenteric plexus and nerve fibers of the circular muscle layer, whereas mutant homozygous mice lacked NOS in both locations.
Applying these observations to the human condition, Huang et al hypothesized that the stomach and pylorus may be particularly dependent on NO and prone to dysfunction in its absence. [4] Although human pyloric stenosis does not appear to be due to a complete absence of neuronal NOS gene product, the absence of NOS in this area may result in pyloric smooth-muscle hypertrophy.
In a 2006 study, Huang et al collected biopsy samples of the pylorus in 13 patients with infantile hypertrophic pyloric stenosis, found decreased expression of neuronal NOS, and demonstrated that plasma nitrite levels can be valuable for diagnosing pyloric stenosis. [5]
With regard to other factors that contribute to smooth-muscle control and hypertrophy, one study of 81 pyloric stenosis pedigrees used single nucleotide polymorphism (SNP)-based linkage analysis to identify two pertinent functional genes on loci 11q14-22 and Xq23. [6] These areas are thought to play a part in the canonical transient receptor potential (TRPC) family of ion channels and may contribute to the development of pyloric stenosis in infants.
Associated anomalies, though rare, have been reported with pyloric stenosis. Approximately 4-7% of infants with pyloric stenosis have associated anomalies, with hiatal and inguinal hernias being the most common. Other anomalies include congenital heart disease, esophageal atresia, tracheoesophageal fistulas, renal abnormalities, rubella, and chromosomal abnormalities such as Turner syndrome and trisomy 18.
In 1993, Jackson et al found that 3.8% of infants (12/308) with de Lange syndrome had pyloric stenosis. [7] Infants with a developmental delay called FG syndrome [8] and those with Smith-Lemli-Opitz (SLO) syndrome, a type of cholesterol deficiency, were reported to be at increased risk for pyloric stenosis. Additionally, Liede et al proposed a convincing argument of a common genetic association between endometriosis, breast cancer, and pyloric stenosis in several families. [9]
Epidemiology
Reports of pyloric stenosis in the United States have cited frequency figures ranging from as low as 1 case per 3000-4000 live births to as high as 8.2-12 cases per 1000 live births. The reported incidence has been decreasing. [10]
This condition is most commonly observed in whites of northern European descent, is less frequently observed in blacks, and is rarely found in patients of Asian or East Indian ancestry. [11, 12] Location also contributes to frequency, with areas where the population is more than two-thirds rural showing an increased risk of 1.79.
Pyloric stenosis is more common in males than in females (male-to-female ratio, 4:1). The highest incidence is in first-born males. A genetic predisposition is suggested in families with occurrences of pyloric stenosis reported in at least three generations. Involvement in twins has been reported, with an 85.7% concordance rate in monozygotic twins and an 8.4% concordance rate in dizygotic twins.
In 1969, Carter and Evans suggested a sex-modified polygenic inheritance of pyloric stenosis. [13] Data from more than 1200 families demonstrated a 20% risk in sons and a 7% risk in daughters of females having had pyloric stenosis, whereas data showed only a 5% risk in sons and a 2.5% risk in daughters of males with pyloric stenosis.
Another report showed a 29% increased risk associated with younger maternal age (< 20 y), whereas a maternal age exceeding 30 years was associated with a significantly decreased risk. [14]
Prognosis
Pyloromyotomy that is adequately performed is curative of pyloric stenosis. There have been reports of pyloric stenosis recurring despite performance of an adequate pyloromyotomy, but recurrence is considered to be a rare exception after incomplete pyloromyotomy has been ruled out.
Yoshizawa et al demonstrated in studies using US imaging that after pyloromyotomy, the pylorus changes significantly within 3 days postoperatively and returns to normal within 5 months. [15] Specific changes include the following:
-
The dorsal pyloric aspect temporarily thickens from 5.1 ± 0.8 mm to 6.0 ± 0.3 mm within 3 days postoperatively and thins out to 2.8 ± 0.2 mm within 5 months
-
The pyloric length decreases from 20.1 ± 2.9 mm preoperatively to 16.9 ± 2.7 mm within 3 days postoperatively and to less than 15 mm within 4 months
-
The change in pyloric diameter is comparable to the change in pyloric length
-
The transverse muscle thickness of the incision site changes from 4.3 ± 0.4 mm to 4.6 ± 0.4 mm within 3 days postoperatively and to 2.1 ± 0.9 mm within 7 days (normal, < 3 mm)
Several studies focused on patient outcomes with respect to advanced training and experience of surgeons. In 2002, Pranikoff et al reported that pediatric surgeons performing pyloromyotomy had a mucosal perforation rate of 0.5%, compared with a 2.9% rate for general surgeons, and that this difference in rate of mucosal perforation correlated with significant decreases in total hospital charges ($4806 ± $79 vs $6592 ± $492) and hospital stay (2.7 ± 0.1 vs 3.1 ± 0.1 d). [16]
In a 2005 study of 11,003 patients with pyloric stenosis, Safford et al stratified patient outcomes on the basis of surgeon volume and hospital volume of pyloric stenosis cases. [17] For surgeons, low volume was considered less than one procedure per year; intermediate volume, one to five procedures per year; and high volume, more than five procedures per year. For hospitals, low volume was considered fewer than five procedures per year; intermediate volume, five to 15 procedures per year; and high volume, more than 15 procedures per year.
In this study, patients operated on by low- and intermediate-volume surgeons were more likely to have complications than those operated on by high-volume surgeons. [17] Patients operated on at low-volume hospitals were 1.6 times more likely to have complications than those at intermediate- or high-volume hospitals. Procedures performed at high-volume hospitals were less expensive than those done at intermediate-volume hospitals, by a margin of $910. High-volume surgeons were more expensive than low-volume surgeons, by a margin of $511. Low-volume surgeons at low-volume hospitals had mucosal perforation rates 4-6.7 times higher than high-volume surgeons at high-volume hospitals.
It is important to note that between 1994 and 2000, the frequency of laparoscopic pyloromyotomy was likely increasing; however, the rates of open pyloromyotomy and laparoscopic pyloromyotomy were not included in procedure coding. The data have shown that for pyloromyotomy procedures, complication rates are lower and cost savings greater with high-volume surgeons operating at high-volume hospitals.
Laparoscopic pyloromyotomy has a significant learning curve. In 2005, Hendrickson et al reported an initial operating time of 70 minutes at a teaching hospital, with operating times decreasing to 15 minutes after 25 procedures. [18] A conversion rate of 8% from the laparoscopic to the open procedure was reported. Similar learning curves were reported at other centers. It has been suggested that appropriate simulation models may help shorten the learning curve for laparoscopic pyloromyotomy. [19, 20]
In 2004, Yagmurlu et al compared open pyloromyotomy (n = 225) with laparoscopic pyloromyotomy (n = 232) and found the overall complication rates to be 4.4% for the open procedure and 5.6% for the laparoscopic procedure. [21] The open approach resulted in a higher rate of mucosal perforation (3.6% vs 0.4%), and laparoscopy had a higher rate of postoperative complications, such as incomplete pyloromyotomy (0% for open vs 2.2% for laparoscopic).
In a 2002 retrospective study, Campbell et al reported on 117 patients showing a trend toward significantly higher complication rates with laparoscopic pyloromyotomy than with the open procedure (18% vs 12%). [22] Additionally, significantly higher hospital costs were associated with the laparoscopic approach.
Also in 2002, the International Pediatric Endosurgery Group noted in their guidelines for infantile hypertrophic pyloric stenosis that laparoscopic pyloromyotomy provided cost savings, decreased operating room time, reduced tissue trauma, and improved cosmetic outcome. [23] (As of March 2023, these guidelines were being revised.)
Oomen et al concluded that laparoscopy might be acknowledged as the standard of care if the major postoperative complication rate is low. [24] To achieve this, laparoscopic pyloromyotomy should be performed by pediatric surgeons with specific expertise in this procedure.
Studies from 2018 concluded that laparoscopic pyloromyotomy for hypertrophic pyloric stenosis yielded outcomes equivalent or superior to those of open pyloromyotomy, with lower complication rates and shorter hospital stays. [25, 26] A study from 2021 found no significant differences between the two approaches with respect to duration of surgery, postoperative complications, duration of hospitalization, and weight at the time of surgery. [27] There may be a small increase in the risk of mucosal perforation after laparoscopic pyloromyotomy as compared with open pyloromyotomy. [28]
-
Diagram of anatomic changes associated with pyloric stenosis.
-
Technique used for examining infant with pyloric stenosis. Infant is best examined from right, with mild pressure applied by using first three fingers of right hand in cephalad direction. Careful examination reveals oblong, smooth, hard mass that is 1-2 cm in length. This mass is hypertrophied pylorus and is commonly referred to as olive.
-
Upper gastrointestinal study used for diagnosing pyloric stenosis.
-
Longitudinal ultrasonogram of pyloric stenosis. Pyloric stenosis is diagnosed by demonstration of elongated sausage-shaped mass with pyloric diameter greater than 14 mm, muscular thickness greater than 4 mm, and length of more than 16 mm.
-
Open pyloromyotomy.
-
Laparoscopic pyloromyotomy.
-
Laparoscopic pyloromyotomy. Pyloric incision.
-
Laparoscopic pyloromyotomy. Spreading of incision.