Background
Pulmonary artery banding (PAB) is a technique of palliative surgical therapy used by congenital heart surgeons as a staged approach for operative correction of congenital heart defects. This technique was widely used in the past as an initial surgical intervention for infants born with cardiac defects characterized by left-to-right shunting and pulmonary overcirculation. Within the last two decades, early definitive intracardiac repair has largely replaced palliation with PAB. This trend has evolved because many centers have demonstrated improved outcomes with primary corrective surgery as an initial intervention in the neonate with congenital heart disease. Although the use of PAB has significantly decreased, it continues to maintain a therapeutic role in certain subsets of patients with congenital heart disease.
The primary objective of performing PAB is to reduce excessive pulmonary blood flow and protect the pulmonary vasculature from hypertrophy and irreversible (fixed) pulmonary hypertension. More recently, PAB has played a role in the preparation and "training" of the left ventricle (LV) in patients with dextro-transposition of the great arteries (d-TGA) who are evaluated for a delayed arterial switch procedure. It has found a similar role in training the LV in patients with levo-transposition of the great arteries (L-TGA) who may also be candidates for an arterial switch procedure.
History of the Procedure
The first description of pulmonary artery banding (PAB) in the literature was a report by Muller and Danimann at the University of California, Los Angeles (UCLA) in 1951. [1] In this report, Muller and Danimann described palliation by the "creation of pulmonary stenosis" in a 5-month-old infant who had a large ventricular septal defect (VSD) and pulmonary overcirculation. Following this report, multiple studies were published demonstrating the effectiveness of this technique in infants with congestive heart failure caused by large VSDs, complex lesions (eg, atrioventricular canal defects), and tricuspid atresia. [2, 3, 4, 5, 6, 7, 8, 9, 10, 11]
Although the use of PAB has declined, it remains an essential technique for comprehensive surgical treatment in patients with congenital heart disease. PAB is a palliative but not a curative surgical procedure.
Pathophysiology
Congenital heart defects with left-to-right shunting and unrestricted pulmonary blood flow (PBF) due to a drop in pulmonary vascular resistance result in pulmonary overcirculation. In the acute setting, this leads to pulmonary edema and congestive heart failure in the neonate. Within the first year of life, this unrestricted flow and pressure can lead to medial hypertrophy of the pulmonary arterioles and fixed pulmonary hypertension. Pulmonary artery banding (PAB) creates a narrowing, or stenosing, of the main pulmonary artery that decreases blood flow to the branch pulmonary arteries and reduces PBF and pulmonary artery pressure. In patients with cardiac defects that produce left-to-right shunting, this restriction of PBF reduces the shunt volume and consequently improves both systemic pressure and cardiac output. A reduction of PBF also decreases the total blood volume returning to the LV (or the systemic ventricle) and often improves ventricular function.
PAB may not be tolerated in patients who have cardiac defects that depend on mixing of the systemic and pulmonary venous blood to maintain adequate systemic oxygen saturations. This is particularly true if a restrictive communication is present between the two atria. Therefore, ensuring that such patients have an unrestricted atrial communication is important to allow adequate mixing at the atrial level before proceeding with PAB. This can be accomplished with a balloon atrial septostomy or an operative atrial septectomy at the time of PAB.
Indications
Patients who are selected for pulmonary artery banding (PAB) and staged cardiac repair are determined based on the experience and training of the pediatric cardiologists and congenital heart surgeons at any given institution. Most of these patients fall into two broad categories: (1) those with pulmonary overcirculation and left-to-right shunting who require reduction of pulmonary blood flow (PBF) as a staged approach to more definitive repair and (2) those with transposition of the great arteries (TGA) who require training of the left ventricle (LV) as a staged approach to the arterial switch procedure.
Patients in the first category who are considered for PAB include those with the following diagnoses:
-
Multiple muscular ventricular septal defects (VSDs) with a "Swiss cheese" septum that is technically difficult to repair in the neonate or requires a ventriculotomy
-
Single or multiple VSDs with coarctation of the aorta or interrupted aortic arch, or contraindications to primary repair, including very low birth weight, major extracardiac conditions, major chromosomal abnormalities, pneumonia, recovering from shock, sepsis, multisystem organ failure, and intracranial hemorrhage
-
Single ventricle defects (eg, tricuspid atresia) that are associated with increased PBF in the neonate – Some surgeons may elect to perform PAB concomitantly with arch repair if there is distal arch obstruction and coarctation of the aorta, and if the subaortic area, aortic valve, and ascending aorta are felt to be adequate to support the systemic circulation. This may be an option in the setting of unbalanced atrioventricular septal defect, isomerism, and double outlet right ventricle with mitral atresia. However, there is a high unplanned reoperation rate with this strategy, including conversion to the Norwood procedure. [12]
-
Unbalanced atrioventricular canal (AVC) defects in which the LV is hypoplastic but the potential exists for a 2-ventricle repair with further growth and development [13]
-
Cardiac defects that require a homograft conduit (eg, D-TGA with subpulmonic stenosis requiring a Rastelli-type repair) for complete repair: Use of PAB may allow time for growth of the patient before the complete repair. Interim growth of the patient permits placement of a larger conduit at the time of repair and potentially increases the longevity of the conduit and length of freedom from reoperation. With current clinical practice, most patients with D-TGA pulmonary stenosis (PS) undergo a Rastelli procedure and placement of a right ventricle (RV)–to–pulmonary artery (PA) conduit. If a staged repair is indicated, a PAB is not usually performed because of already decreased PBF. In this situation, a systemic-to-pulmonary shunt is performed. Patients with dextro-transposition of the great arteries (D-TGA) with subpulmonic stenosis usually have decreased PBF. The patient who would require PAB in this situation to allow for somatic growth so as to allow a larger conduit to be placed would be rare.
Patients in the second category who are considered for PAB include those with the following diagnoses:
-
D-TGA that requires preparation of LV for an arterial switch procedure following initial late presentation or diagnosis in patients older than 1 month or older than about 6-8 weeks of age with signs of LV deconditioning.
-
D-TGA that requires preparation of LV for an arterial switch procedure following a previous Mustard or Senning procedure with the development of right ventricular failure or L-TGA that requires preparation of the LV prior to the double switch procedure. PAB in patients with corrected TGA will cause the morphologic RV to become less spherical. There will be a shift of the interventricular septum such that there is improved coaptation of the septal leaflet of the tricuspid valve and an improvement in tricuspid regurgitation. Some groups have shown that PAB for preparation of the morphologic RV in corrected TGA may offer satisfactory long-term palliation in this complex group of patients. [14, 15]
Note that patients with single ventricle physiology and unrestricted PBF are suitable for an early PAB to prevent development of congestive heart failure and pulmonary hypertension. This group of patients may include those who have tricuspid atresia with unrestrictive VSD, unbalanced AVC defect, and double inlet LV. [16] In one reported series, 9 of 20 patients with double inlet LV demonstrated severe PA medial hypertrophy on histologic examination within one year of life. [17] Generally, patients who have single ventricle physiology and pulmonary overcirculation should undergo PAB in the first 1-2 months of life to avoid irreversible pulmonary hypertension that may complicate or preclude a subsequent Fontan procedure.
Currently, most patients with D-TGA undergo an arterial switch procedure within the first few weeks of life. However, some newborns with D-TGA and an intact ventricular septum may not undergo an early arterial switch procedure because of active infections, coexistent noncardiac diseases, or a delay in diagnosis.
Because of the risks of neonatal repair, neonates with D-TGA and multiple VSDs may benefit from bilateral PAB prior to definitive repair later in infancy. This technique may be less prone to damaging the neoaortic valve and root dilation than banding of the main pulmonary artery. [18] Patients with Taussig-Bing syndrome who are premature and have low birth weight, as well as have associated anomalies including arch obstruction, chromosomal and major extracardiac anomalies, pneumonia, shock, hepatic and/or renal failure may also benefit from PAB prior to complete repair. The arch repair and PAB may be done initially, followed by arterial switch and VSD repair in 2-4 weeks.
In the past, patients who did not undergo early an arterial switch procedure were treated by a Mustard or Senning procedure because the arterial switch was precluded by rapid involution of the left ventricular myocardium. Subsequent experience demonstrated that neonatal PAB and concomitant systemic-to-PA shunt resulted in preservation of the LV and reversal of any attenuation of the myocardium, leading to successful arterial switch later in infancy. [19, 20]
PAB is also used in patients with D-TGA who develop right ventricular dysfunction after a Mustard or Senning atrial switch procedure. The PAB is required for a longer period than preparation of the ventricle in infants (< 12 months). Although the overall early survival rate approaches 90%, approximately one half of these patients require heart transplantation because of the progression of coexisting left ventricular failure. [21] Furthermore, a high prevalence of significant neo-aortic valve insufficiency is noted in patients who successfully undergo the arterial switch procedure.
Application of PAB has been reported in patients with diagnosis of L-transposition or physiologically corrected transposition of the great arteries. [22] This group of patients may present with failing systemic RV. Using the same principle, the PAB is used to retrain the LV in preparation for a double switch operation, a combination of an atrial and arterial switch. [23, 24, 25] This operation places the LV as the systemic ventricle and the mitral valve as the systemic AV valve. This achieves anatomic repair of the malformation.
The authors have found another application of PAB in patients with elevated, but reactive, pulmonary hypertension from long-standing left-to-right shunting. An immediate surgical repair may carry significant morbidity and even mortality. With the use of a PAB and pulmonary vasodilator, some of these patients may drop their pulmonary vascular resistance and subsequently respond more favorably to surgery. This approach has been used at the authors' institution with good results. [26]
Relevant Anatomy
In most patients with cardiac defects requiring pulmonary artery banding (PAB), the length of main pulmonary artery (MPA) is sufficient to allow placement of the band in the mid portion of the artery without impingement on either the pulmonary valve, coronary arteries proximally or the branch pulmonary arteries distally. The inferior wall of the right pulmonary artery (PA) arises slightly more proximal on the MPA than the left PA. The right PA also arises from the MPA at more of an acute angle. Both of these factors increase risk of right PA impingement by a distally placed band. The tissue connecting the aorta and MPA in the aortopulmonary window usually must be divided with surgical dissection.
In patients with pulmonary overcirculation, the MPA may be quite large compared to the aorta. Additionally, the MPA vessel wall may be thinned out by this dilatation, and the adventitia may be quite attenuated. These changes increase risk of tearing the wall of the MPA at the time of PAB.
Contraindications
Patients who have single ventricle defects in which the aorta arises from an outflow chamber (eg, double inlet left ventricle [LV], tricuspid atresia with transposition of the great arteries [TGA]) have the potential for development of significant subaortic obstruction. The risk is higher when these lesions are also associated with aortic arch anomalies. [27] Pulmonary artery banding (PAB) is contraindicated in the presence of such obstruction and in patients who are at high risk for such obstruction. The ventricular hypertrophy that develops in response to PAB may cause rapid progression of subaortic obstruction leading to a combination of both ventricles having outflow tract obstruction and progressive hypertrophy.
These patients are identified by careful preoperative assessment, including echocardiography and, if necessary, cardiac catheterization with pullback pressure measurements across the subaortic region. The presence of a gradient more than 15-20 mm Hg or an echocardiographic outlet foramen area index of less than 2 cm2/m2 precludes PAB. Isuprel challenge at the time of cardiac catheterization may expose a gradient across the bulboventricular foramen. The bulboventricular foramen index is described by Matitiau et al. [28]
Instead, these patients should undergo the Damus-Kaye-Stansel procedure and a systemic-to-pulmonary artery shunt. [29, 30] This achieves adequate pulmonary blood flow (PBF) with protection of the pulmonary vasculature and bypasses the subaortic obstruction. Another well-described complication of PAB is the development of subaortic obstruction from conal hypertrophy, particularly in patients with a single ventricle and a subaortic outflow chamber. [31] It may also result from hypertrophy of an abnormally positioned moderator band.
The development or persistence of subaortic stenosis post–PAB can adversely affect outcome of future Fontan procedures through the development of ventricular hypertrophy and consequent subendocardial ischemia. [32] Indeed, the duration of PAB may be an independent risk factor for a subsequent Fontan procedure. [33] If obstruction occurs later, the authors perform a resection of the obstruction or a Damus-Kaye-Stansel procedure, with or without a concomitant Fontan procedure. Earlier cavopulmonary connection may be warranted when anatomy and physiology are appropriate. The operative mortality rate with early intervention remains comparable to the overall group of patients who undergo the Fontan procedure. [34]
PAB is not used in patients diagnosed with truncus arteriosus. Although a main pulmonary artery (MPA) is present in truncus arteriosus type I, it usually is very short and does not allow for successful PAB without impingement on the right pulmonary artery (PA) or the origin of the MPA from the truncal artery. In truncus arteriosus types II and III, bilateral PAB is necessary to effectively reduce PBF. [35] Previous experience has shown that balancing PBF to the right and left lungs is extremely difficult. Furthermore, subsequent complete repair is complicated by bilateral PA stenosis requiring extensive reconstruction. For these reasons, PAB is avoided in this group of patients.
Bilateral PAB may be useful prior to complete repair in the setting of low birth weight, prematurity, major associated extracardiac conditions, severe preoperative acidosis not correctable by medical therapy, pneumonia, as a staged repair in truncus arteriosus with interrupted aortic arch, and/or a combination of these factors. Bilateral PAB can be achieved using a 3.0-mm or 3.5-mm Gore-tex graft placed around the right and left pulmonary arteries as has been described for hypoplastic left heart syndrome (HLHS). [36] Bilateral PAB has also been used as a bridge to decision regarding biventricular versus univentricular palliation. For example, bilateral PAB with maintenance of ductal patency may allow time for adequate growth of a left ventricle so that the infant can undergo biventricular repair. The pulmonary artery bands can also be dilated in case of desaturation, to allow further time before definitive surgery. [37]
Note that early attempts to use PAB in surgical management of HLHS were also unsuccessful. [38] However, more recent reports have shown that, in high-risk patients with HLHS, a hybrid approach of stenting the ductus arteriosus and bilateral PAB may achieve effective short-term palliation. [39, 40, 41, 42, 43, 44] As with the early attempts at PAB in patients with truncus arteriosus, balancing the systemic and pulmonary blood flow and achieving near-equal distribution of blood flow to the right and left lungs can be extremely difficult in these patients. This is a technically delicate and demanding maneuver that, until more data are available, should only be considered in high-risk patients.
As discussed above, bilateral PAB can be achieved using a 3.0-mm or 3.5-mm Gore-tex graft placed around the right and left pulmonary arteries as has been described for HLHS. [36] This group and others have reported a high success rate with the hybrid procedure in HLHS. [45]
-
Pulmonary Artery Banding. The left anterior thoracotomy approach through the second or third intercostal space gives excellent exposure for isolated pulmonary artery banding. Note anatomy of the adjacent structures with medial limits of the incision at the internal mammary vessels. The thymus is swept superiorly away from the phrenic nerve. PA = pulmonary artery; PDA = patent ductus arteriosus.
-
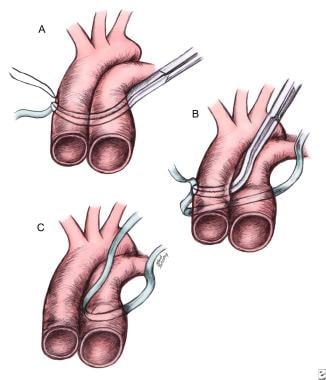
Pulmonary Artery Banding. Safe placement of a pulmonary artery band: (A) encircling the aortopulmonary trunk, (B) encircling the aorta, and (C) completing the pulmonary artery band at the final location.
-
Pulmonary Artery Banding. Pulmonary artery banding technique (A-C) using a premeasured Trusler formula adjusted to the cardiac anatomy and physiology. An adjustable device is placed over a felt pledget with adventitial fixation sutures to prevent distal migration. Additional fixation sutures may be placed in the band itself. Each additional medium hemoclip causes approximately 1 mm of change in band circumference. Distal pulmonary artery pressure is measured during tightening.
-
Pulmonary Artery Banding. Circumferential banding of a dilated pulmonary artery can acutely lead to internal infolding of the arterial wall. Later resorption of the infoldings and remodeling of the arterial wall restore a greater internal cross-sectional area.
-
Pulmonary Artery Banding. Incisional pulmonary artery band yields a fixed reduction of 40% of the vessel diameter before application of a circumferential band.
-
Pulmonary Artery Banding. (A) A partial Senning technique for improved mixing and saturation and providing both volume and afterload to the left ventricle after pulmonary artery banding. Autologous pericardium is used to baffle the inferior vena cava and the left pulmonary veins across the mitral valve into the left (pulmonary) ventricle, with superior vena cava and right pulmonary vein drainage to the systemic ventricle. The band is best positioned distally to avoid valvular damage in anticipation of the arterial switch procedure. (B) Pulmonary artery banding combined with a modified Blalock-Taussig shunt for rapid preparation of the left ventricle and the arterial switch procedure. This provides left ventricular volume and afterload and can be performed through a left lateral thoracotomy, avoiding the need for a resternotomy at the time of arterial switch. Careful measurement of the proximal pulmonary artery pressure is shown to avoid overtightening the band. PA = pulmonary artery.
-
Pulmonary Artery Banding. Reconstruction of the pulmonary artery after band removal may be accomplished by patch arterioplasty using glutaraldehyde-treated autologous pericardium (A) or polytetrafluoroethylene (PTFE) material (B) or resection of the band site and end-to-end anastomosis using absorbable running sutures (C).