Overview of Pediatric Lung Transplantation
Lung transplantation has matured into an accepted therapeutic alternative for children with end-stage lung disease. From 1986 through June 2016, 2330 lung and 730 heart-lung transplants in children were reported to the Registry for the International Society for Heart and Lung Transplantation (ISHLT). Following a steady increase in pediatric lung transplants in the first decade of this millennium, with 125 reported to the ISHLT registry in 2009, numbers have remained stable with a range of 90 to 137 observed during the past 12 years. [1]
However, the phrase “children are not just small adults” is nowhere more true than in the field of lung transplantation. The indications for transplantation, the pharmacokinetics of immunosuppressants, and the complications of transplantation can be strikingly different for children compared with their adult counterparts. This article is meant not only to serve as an overview of the field of lung transplantation but also to highlight the unique challenges faced by pediatric lung transplant recipients, their families, and their healthcare teams. [2]
By its nature, transplantation implies life-long medical management. Transplantation is not a cure; it is a trade. That is, patients trade their end-stage lung disease for transplant lung disease, with the hope that it can be better managed.
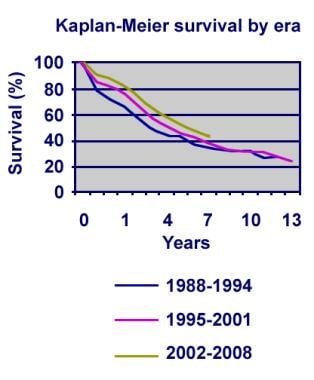
Although one-year survival rates have improved, long-term outcomes for children receiving lung transplants have not (see the image below). Various imposing obstacles keep physicians from obtaining the long-term results they seek. Rejection and, specifically, bronchiolitis obliterans (BO) remains a major hurdle to overcome. A better understanding of the underlying mechanism(s) that leads to BO is crucial. [3]
The development of improved immunosuppressants or, better yet, the development of agents that allow for immunologic tolerance would certainly provide improved clinical outcomes and potentially reduce the risk of infection. Improved understanding of the mechanism of lung injury during brain death and during transition from donor to recipient may one day lead to techniques and preservation solutions that prevent ischemia-reperfusion injury. [4]
Better strategies must be developed to address the emotionally incapacitating state of adolescence that often leads to poor adherence to an admittedly complicated medical regimen. [5] Finally, the shortage of organ donors must be addressed because too many patients die on the waiting list and never receive a second chance at life.
For patient education resources, see the Lung and Airway Center, as well as Heart and Lung Transplant and Bronchoscopy.
History of Lung Transplantation
In 1963, James Hardy, MD, at the University of Mississippi, performed the first human lung transplantation in a 58-year-old convict serving a life sentence. The patient presented with a squamous carcinoma at the left hilum. During surgery, he was also found to have an empyema. He survived for 18 days after transplantation and died of renal failure. His immunosuppression consisted of azathioprine, prednisone, and radiation therapy. Despite an ABO mismatch, no rejection was found during autopsy.
The procedure evolved over subsequent decades, with changes in the surgical technique and the immunosuppressive regimens. Joel Cooper, MD, at the University of Toronto achieved success in 1983 with the transplantation of a single lung. The recipient lived for 6.5 years. This procedure was soon followed by successful double lung transplantation. Lastly, Bowdish et al and other groups demonstrated that living-donor lobar lung transplantation can be successfully performed. [6]
Frequency of Pediatric Lung Transplantation
According to the International Society of Heart and Lung Transplantation (ISHLT) Registry, 2,330 pediatric lung and 730 pediatric heart-lung transplants have been performed through June 30, 2016. Most of the lung transplants reported for 2015 were performed in older children (aged 11–17 years), with 61% of children who received an allograft aged ≥ 11 years and only 1 lung transplant in a child aged < 1 year. [1]
In 2022, there were 18 lung transplants and 3 heart-lung transplants in patients younger than 18 years, and 31 patients younger than 18 years were on the United Network for Organ Sharing (UNOS) waiting list for either lung or heart-lung transplants. [7]
Indications for Lung Transplantation
In patients with untreatable end-stage lung disease due to various etiologies, lung transplantation warrants consideration.
In children younger than 1 year, the most common causes of end-stage disease include the following:
-
Congenital heart disease (CHD)
-
Surfactant dysfunction syndromes
-
Idiopathic pulmonary hypertension ( IPH) and other pulmonary vascular disorders
In children aged 1-10 years, end-stage lung disease due to cystic fibrosis (CF) results in over 50% of all lung transplants. In adolescents, that number increases to almost 70%. [1]
Determining which children may benefit from lung transplantation is often not straightforward; the correct timing of the referral to a transplant center is also difficult to determine. The decision to perform transplantation is based on many factors, including past experience (both center-specific and that in published reports), the scarcity of donor organs, and the specific wishes of patients and their parents or guardians.
Indications for lung transplantation in children have expanded, and referral to a transplant center should be considered in virtually any child with a life expectancy that is limited because of lung disease. However, exactly when lung transplantation should be considered during the specific disease process requires detailed attention not only to the trajectory of the underlying illness but also to psychosocial factors, such as the emotional readiness of the patient for the demands of daily therapy and frequent procedures. [8]
The prognosis of the patient’s natural disease must first be made clear. Lung transplantation is a procedure of last resort and, when performed, should ideally provide the patient an improved likelihood of survival and an improved quality of life.
Cystic fibrosis
Investigators have developed different models that attempt to predict survival in patients with CF. Initially, according to Kerem’s criteria, a forced expiratory volume in 1 second (FEV1) of less than 30% was associated with an increased death risk within 2 years; associated factors include decreased arterial oxygen tension (PaO2), an increase in arterial carbon dioxide tension (PaCO2) on room-air arterial blood gas (ABG) findings, younger age, and female sex. Other investigators later found that this model alone did not reliably predict 2-year survival in their patient populations.
In 2001, Liou and colleagues developed a model to predict the 5-year survival rate. [9] The key clinical predictors of increased mortality rates included increasing age, female sex, diabetes mellitus, airway infection due to Burkholderia cepacia, [10] and frequent acute exacerbations. In contrast, an increased FEV1, increased weight-for-age Z score, pancreatic sufficiency, and airway infection due to Staphylococcus aureus seemed to have a protective effect on survival.
However, this model was limited to information collected by the Cystic Fibrosis Foundation Patient Registry and, thus, did not take into account other factors that many clinicians routinely consider in evaluating a patient with CF for lung transplantation, which include the following:
-
Room-air ABG findings
-
Emergence of resistant organisms
-
Degree of exercise intolerance
-
Presence of cor pulmonale
This model was then used to assess transplant benefit. [11] The authors designed a proportional hazards model to specifically measure the clinical benefit of lung transplantation in children with CF. [12] The model and its result sparked much controversy and debate (see Outcomes After Lung Transplantation). [13, 14]
In 2015, the International Society for Heart and Lung Transplantation (ISHLT) published an update to its consensus document for the selection of lung transplant candidates, which concludes that transplantation indications are a 2-year predicted survival of < 50% and functional limitations classified as New York Heart Association (NYHA) class III or IV. [15]
The ISHLT uses the following criteria to determine timing of referral [15] :
-
FEV 1 that has fallen to 30% or, in a patient with advanced disease, a rapidly falling FEV 1 despite optimal therapy (particularly in a female patient), infection with non-tuberculous mycobacteria (NTM) or B cepacia complex (see previous comment on B cenocepacia and subsequently) and/or with diabetes
-
A 6-minute walk distance < 400 m
-
Development of pulmonary hypertension in the absence of a hypoxic exacerbation (as defined by a systolic pulmonary arterial pressure (PAP) > 35 mm Hg on echocardiography or mean PAP > 25 mm Hg measured by right heart catheterization)
-
Clinical decline characterized by increasing frequency of exacerbations associated with any of the following:
- An episode of acute respiratory failure requiring non-invasive ventilation
- Increasing antibiotic resistance and poor clinical recovery from exacerbations
- Worsening nutritional status despite supplementation
- Pneumothorax
- Life-threatening hemoptysis despite bronchial embolization
The ISHLT uses the following criteria to determine timing of listing [15] :
-
Chronic respiratory failure (CRF)
-
CRF wth hypoxia alone (partial pressure of oxygen [PaO 2] < 8 kPa or < 60 mm Hg)
-
CRF with hypercapnia (partial pressure of carbon dioxide [Paco2] > 6.6 kPa or > 50 mm Hg)
-
Long-term non-invasive ventilation therapy
-
Pulmonary hypertension
-
Frequent hospitalization
-
Rapid lung function decline
-
World Health Organization Functional Class IV
Pulmonary vascular disease
In children, pulmonary vascular disease may be due to several etiologies, including idiopathic pulmonary hypertension (IPH), CHD, and pulmonary vein anomalies. [16] Therapeutic advances have dramatically changed outcomes for children with IPH. Therefore, in children with IPH, transplantation should be considered only when medical therapy fails.
Guidelines have been published for adults in whom medical management fails; NYHA class III or IV, low exercise tolerance, uncontrolled syncope, hemoptysis, and right heart failure are useful parameters. Whether these guidelines are appropriate for children is unclear.
In children, a decreased cardiac index, elevated pulmonary vascular resistance, right atrial pressures of more than 7.4 mm Hg, and right ventricular end-diastolic pressure of more than 10.4 mm Hg portend a poor survival and are indications for lung transplantation. Other factors that may be correlated with survival include von Willebrand factor levels, elevated uric acid levels, and plasma levels of brain natriuretic peptide.
Surfactant dysfunction syndromes
Surfactant dysfunction syndromes are associated with congenital alveolar proteinosis. The spectrum of presentation varies, with some children presenting with respiratory failure within hours of birth, and others presenting with only subtle symptoms later in life. For a comprehensive review of this topic as it relates to lung transplantation, see the article entitled “Lung transplantation for inherited disorders of surfactant metabolism” by Faro and Hamvas (2008). [17]
Bronchopulmonary dysplasia
Because patients with bronchopulmonary dysplasia (BPD) severe enough to merit consideration of lung transplantation often have associated comorbidities, BPD is a fairly uncommon indication for lung transplantation. In addition, many infants with BPD improve if given the opportunity to grow. Lung transplantation is an option for children with BPD who:
-
Have no significant comorbidities
-
Are on ventilatory support
-
Are not showing clinical improvement
-
Have evidence of pulmonary hypertension that is unresponsive to oxygen therapy.
Ultimately, the physician who determines the timing of referral should consider the patient’s assessment of quality of life while remembering the ideal transplant window, during which the patient is medically ill enough to warrant transplantation but not quite so ill that the odds of a good outcome are unacceptably poor.
Early referral to a transplant center is preferable to late referral. This provides the center the opportunity to establish a relationship with the child and family. In addition, it allows the patient time to truly understand the commitment that lung transplantation requires and allows the center to put into place any necessary measures necessary to ensure an acceptable outcome.
Contraindications to Lung Transplantation
Contraindications to lung transplantation vary from center to center. [15] An absolute contraindication at one site may be only a relative contraindication at another. This is especially true for airway infections. More importantly, the list is not static; it changes over time with the introduction of new techniques or therapies.
The following are the absolute and relative contraindications currently recognized by most, but not all, pediatric centers.
Absolute contraindications include the following:
-
Active malignancy
-
Sepsis
-
Cerebral dysfunction
-
Severe neuromuscular disease
-
Documented refractory nonadherence
-
Active hepatitis C infection
-
Multiorgan dysfunction
Relative contraindications include the following:
-
Pleurodesis
-
Renal insufficiency
-
Markedly abnormal body mass index
-
Mechanical ventilation [20]
-
Severe scoliosis
-
Poorly controlled diabetes mellitus
-
Active collagen vascular disease
-
Congenital or acquired immunodeficiency syndromes
Clinical Presentation of Pediatric Candidates
The clinical presentation of children who present for an evaluation for lung transplantation varies depending on the primary diagnosis. However, all these children have end-stage lung disease with an impaired quality of life.
Children may present with the following:
-
Respiratory insufficiency or respiratory failure
-
Exercise intolerance
-
Poor growth
-
Hypoxemia
-
Carbon dioxide retention
-
Abnormal pulmonary function test findings
-
Frequent respiratory exacerbations that require antibiotics and/or anti-inflammatory medications.
Children with underlying pulmonary vascular disorders may present with the following:
-
Exercise intolerance
-
Poor growth
-
Cyanosis
-
Impaired quality of life
Workup of Pediatric Candidates
Laboratory studies
Laboratory tests to be considered in evaluating pediatric patients for lung transplantation include the following:
-
Complete blood count (CBC) with differential - To establish baseline values and screen for underlying immunodeficiency
-
Prothrombin time (PT) and/or activated partial thromboplastin time (aPTT) - To detect abnormalities that may complicate surgery if untreated
-
Blood typing and screening - To match the donor and recipient
-
Renal disease battery - To detect adverse effects of calcineurin inhibitors and certain antimicrobial agents
-
Liver function tests and/or hepatitis battery - To assess for contraindications (ie, abnormal results) to lung transplantation
-
Preformed reactive antibody panel - To assess the risk of development of antibody-mediated rejection
-
Lipid profile - To detect adverse effects of certain immunosuppressant agents
-
Human immunodeficiency virus (HIV), venereal disease research laboratory (VDRL) test - To evaluate for a contraindication (ie, abnormal results) to lung transplantation
-
Immunoglobulin G (IgG) serologic tests for rubeola, rubella, herpes, Epstein-Barr virus (EBV), varicella, toxoplasmosis, and cytomegalovirus (CMV) [21] - To screen for previous exposure and the need for vaccination when appropriate and to determine the risk of disease or its reactivation
-
Autoimmune screening for antinuclear antibodies (ANA), antinuclear cytoplasmic antibodies (ANCA), rheumatoid factor, and quantitative immunoglobulins (in specific patients)
-
Arterial blood gas (ABG) tests - To provide a measure of lung function
-
Thyroid profile
Imaging studies
Imaging studies that may be ordered include the following:
-
Chest radiography - To evaluate the extent of disease
-
Chest computed tomography (CT) scanning - To evaluate the extent of disease and to determine the size of the thorax and vessels
-
Ventilation-perfusion scanning - To assist in determining the function of both lungs and, in a bilateral sequential procedure, to determine which lung should be replaced first
-
Echocardiography - To evaluate for pulmonary hypertension
-
Sinus CT scanning - To determine the need for surgical intervention in patients with cystic fibrosis (CF) before transplantation (because the sinuses contain organisms that may reinfect the lower respiratory tract after transplantation)
-
Bone densitometry - To assess risk for fractures in patients with end-stage lung disease. (These patients often have a history of steroid use and are at risk for fractures [22] ; measuring bone density allows for appropriate interventions to be put in place.)
Other tests
The following tests may also prove helpful:
-
Pulmonary function testing - To help determine the need for lung transplantation
-
Six-minute walk test - To help determine the need for lung transplantation
-
Sputum culture and susceptibilities - To direct the choice of antimicrobial agents after transplantation. (Some centers may perform further susceptibility testing to determine combinations of antibiotics that may be synergistic; however, the available data suggest that this information may not be useful.)
-
Purified protein derivative (PPD) of tuberculin skin test - To rule out active tuberculosis, which is an absolute contraindication to lung transplantation
-
Electrocardiography - To screen for right ventricular hypertrophy or other cardiac dysfunction
-
Cardiac catheterization - In select cases, to measure degree of pulmonary hypertension or to assess benefits of vasodilator therapy
Diagnostic procedures
Bronchoscopy with bronchoalveolar lavage may be indicated to isolate pathogens from the lower airways or to document clearance of atypical mycobacteria. A lung biopsy should be considered if the diagnosis and prognosis remain unclear.
Preoperative Details
Optimization of medical and psychosocial care
Treatment varies depending on the primary diagnosis. The goal of therapy for patients on the transplant waiting list is to optimize their medical care in preparation for the upcoming surgery and to correct deficiencies discovered during the evaluation. This approach may include improving the patient’s nutritional status, providing pulmonary rehabilitation, and trying to decrease the number of pulmonary exacerbations that require intravenous antibiotics.
Advances in the treatment of pulmonary vascular disease have changed the outlook for many patients with idiopathic pulmonary hypertension (IPH). In some patients with IPH, medical therapy with epoprostenol has delayed or obviated lung transplant. Other pulmonary vasodilators, such as sildenafil and bosentan, have also been effective in treating these patients.
Extracorporeal membrane oxygenation (ECMO) can be used to bridge patients with end-stage lung disease to lung transplantation. Tipograf et al reported that when ECMO is implemented by an experienced team with adherence to stringent protocols and patient selection, outcomes are comparable to those in patients who did not receive pre-transplant support. [23]
The Novalung Interventional Lung Assist device, a pumpless venovenous membrane ventilator, is an efficient remover of carbon dioxide. It is used for patients with acute respiratory distress syndrome, as well as other forms of severe acute respiratory failure. Emerging reports suggest that this device may be useful as a bridge to lung transplantation in appropriate candidates with adequate cardiac function. The youngest child placed on the device thus far was a 2-year-old at St. Louis Children’s Hospital with pulmonary hypertension who managed to improve enough to so far avoid the need for a lung transplant.
If the patient has psychosocial contraindications to transplantation, the authors attempt to correct those conditions by providing their families the assistance they need to successfully care for their child. If that assistance is ineffective and if transplantation is otherwise deemed appropriate, one must give careful consideration to involving the appropriate state authorities for alternative placement of the child. However, at some centers, the need for placement is considered a contraindication to transplantation.
Surgical intervention before transplant
Surgical intervention prior to transplant may vary depending on the primary diagnosis. For instance, patients with cystic fibrosis (CF) and end-stage lung disease may develop pneumothoraces. Before the advent of transplantation, these patients routinely underwent pleurodesis to prevent recurrences. However, pleurodesis potentially complicates transplantation surgery and is a relative contraindication.
In fact, before one proceeds with any thoracic procedure, the transplant center should be consulted because such procedures may disqualify the patient from future transplantation surgery. Any thoracic procedure before transplantation may increase the risk of bleeding and intraoperative or immediate postoperative mortality.
Other surgical interventions that may be contemplated prior to transplant include the placement of a gastrostomy tube for nutritional resuscitation.
Allocation of organ to recipient
After a patient is listed for lung transplantation, if they are aged 12 years or older, the timing of the transplantation depends on their lung allocation score (LAS). The LAS was established in 2005. [24] This allocation system factors in the risk of mortality while on the waiting list and the benefit of transplantation. In addition, this system allows pediatric donor lungs to be preferentially offered to pediatric recipients within the region before being offered to a wider pool.
Clinical findings and laboratory values used to determine the LAS include the following:
-
Age
-
Underlying diagnosis
-
Forced vital capacity
-
Oxygen levels at rest
-
Six-minute walk test results
-
Ventilator use
-
Functional status (NYHA class)
-
Systolic pulmonary artery pressure
-
Pulmonary capillary wedge pressure
-
Body mass index
-
Diabetes diagnosis
-
Creatinine levels
In 2010, UNOS adopted a new allocation system, within the framework of waiting time, for children younger than 12 years. It allows for prioritization of lungs to the sickest child by developing 2 distinct priority levels. Priority 1 candidates are those who meet one of the following criteria:
Respiratory failure as defined by one or more of the following:
-
Continuous mechanical ventilation
-
Forced inspiration of oxygen (FiO 2 ) more than 50% to maintain saturation levels of more than 90%
-
An arterial or capillary PCO 2 of more than 50 mm Hg or a venous PCO 2 of more than 56 mm Hg
Pulmonary hypertension as defined by any of the following and on medical therapy:
-
Pulmonary vein stenosis involving 3 or more vessels
-
Suprasystemic pulmonary artery pressures
-
Cardiac index < 2 L/min/m 2
All other patients under 12 years are priority 2 candidates. Donor lungs are first allocated to the child with the most waiting time as a priority 1 candidate who also matches the donor’s blood type and size. If no suitable priority 1 candidates are found, then the lungs are offered to the child with the most waiting time as a priority 2 candidate.
Assessment of potential donor
The potential lung donor should meet certain criteria; however, these criteria are mostly derived from experience and not from controlled trials. [25, 26]
Characteristics of ideal donors include the following:
-
Age 55 years or younger: Data suggest poorer outcomes with older donors, especially when combined with ischemic times longer than 6 hours. [27]
-
Arterial blood gas (ABG) results that include a ratio of arterial oxygen pressure to fraction of inspired oxygen (PaO2/FiO2) of more than 300 mm Hg and a positive end-expiratory pressure (PEEP) of 5 cm water: Data are inadequate to support or refute this cutoff. Studies mainly focus on interventions to increase donor PaO2/FiO2 to more than 300 mm Hg to harvest the organ.
-
Normal chest radiography findings: The use of donors with abnormal chest radiography findings has not been studied. If findings suggest atelectasis, aggressive airway clearance and the use of bronchoscopy to remove mucus plugs may salvage an otherwise unusable lung. However, if findings suggest a contusion, the lung should not be accepted.
-
Ischemic time of 4-6 hours: Reports describe successful outcomes with ischemic times of 6-11 hours. Poor outcomes are associated with the combination of older donors and prolonged ischemic times.
-
Height and/or predicted total lung capacity (TLC) ± 20% of recipient: Although data from adults demonstrate no adverse results on outcomes when using donor lungs within 75-125% of recipient TLC, a UNOS database review by Fraser et al found that in patients younger than 13 years, size mismatching (height, weight, or TLC) is associated with increased mortality; these authors recommend prioritizing well-matched allografts for pre-adolescent patients. [28]
-
Negative Gram stain findings: Infection is a major source of early posttransplant morbidity and mortality; however, evidence suggests that positive sputum Gram stain findings do not correlate with the development of pneumonia. Data suggests that the amount of purulent secretions may be important in predicting posttransplant outcome.
-
A history of smoking less than 20 packs per year: No data support or refute this criterion. Concerns include the development of malignancy posttransplant as well as the potential for increased risk of poor perioperative outcome.
-
No history of cancer: Few data are available regarding donors with a past history of malignancy. The potential risk is likely based on histology, tumor stage, and length of cancer-free survival. Primary central nervous system (CNS) tumors rarely spread. Risk factors for metastases include medulloblastoma or glioblastoma, previous craniotomy, ventricular shunt, and tumor radiation. Renal cell carcinoma is the most common type of cancer transmitted from donors.
-
Mechanical ventilation for less than 3 days: Mechanical ventilation for longer than 2 days is a risk factor for the development of ventilator-associated pneumonia. However, donors with prolonged courses who have normal radiography findings and good gas exchange may be better donors because the sequelae of aspiration may not be evident within the first 24-48 hours.
A comprehensive approach to the management of the prospective pediatric donor has been published. [29]
Intraoperative Details
Sequential bilateral lung transplantation
The most common pediatric surgical technique in the United States now consists of a sequential bilateral procedure with telescoping anastomoses. This procedure may allow the surgeon to avoid the need for cardiopulmonary bypass, reducing the risk of infection and bleeding.
In a sequential bilateral lung transplantation, the surgeon performs a transverse thoracosternotomy, allowing for excellent visualization of the pleural space. The patient’s least functional lung, as determined during the transplantation evaluation, is removed first, while the contralateral lung provides uninterrupted ventilation. The bronchus, pulmonary artery, and pulmonary vein anastomoses are performed, in that order. The bronchial circulation, lymphatic system, and nervous system are not reanastomosed.
Because of the relative difficulties of double-lumen endotracheal tube use in small children and the prevalence of pulmonary hypertension in that cohort, cardiopulmonary bypass is often used in younger children.
Living lobar donation
Living lobar donation allows a family member or friend to donate a lower lobe of his or her lung. [30] This procedure was devised in the era when lungs were allocated strictly based on time spent on the wait list. Some patients presented late in the course of their illness and did not realistically have 2-3 years to wait. In living lobar donation, one donor donates a right lower lobe, and another donates a left lower lobe, allowing patients to circumvent the once-necessary wait list.
Using lungs from living donors offers several potential advantages over using lungs from deceased donors. Most notably, lung transplantation surgery is changed from an emergency procedure to an elective one. The surgery can be scheduled at a time best suited to the requirements of the recipient, donors, and transplantation team, thus potentially decreasing the risk of ischemia-reperfusion injury. However, the procedure involves performing thoracotomies in two healthy donors, placing them at risk for complications.
Because lungs are now allocated based on the lung allocation score rather than time on the list, the most obvious reason for performing living lobar donation (ie, not having enough time accrued on the list) is no longer valid, at least for primary transplantation in which no difference in survival outcomes between the two procedures is noted. This is despite data suggesting that the risk for developing bronchiolitis obliterans (BO) may be lower in the living lobar recipient. [31] Data have shown that reoperative lung transplant recipients who receive living donor donations may have better outcomes than those who receive lungs from a deceased donor. [32]
Postoperative Details
If all goes well, the typical postoperative course consists of a 2-week hospitalization period. The goal is to extubate patients within 24-48 hours of transplantation to minimize barotrauma and further oxidant injury. While intubated, patients may require frequent bronchoscopy to evaluate the anastomotic site and to suction debris and secretions (secondary to the reimplantation response) from the airway. Chest tubes are typically removed when they are draining less than 100 mL of fluid per day and when no air leak is present.
When all pressors are discontinued and extubation has been accomplished, the patient can be transferred from intensive care. Appropriate intravenous antibiotics and other warranted antimicrobials are continued for approximately 2 weeks. Physical therapy is initiated in the intensive care unit and progressively becomes more strenuous as the patient becomes ambulatory.
Discharge to posttransplantation housing varies by center. At St. Louis Children’s Hospital, housing is near the hospital and patients continue to attend regular sessions of physical therapy. One month after surgery, they undergo surveillance bronchoscopy with transbronchial biopsy. The timing of surveillance procedures vary from center to center.
Induction Regimens
A well-established risk factor for the development of bronchiolitis obliterans (BO) is a history of acute rejection. [33] This has led to the institution of induction regimens at many centers. The most recent International Society of Heart and Lung Transplantation (ISHLT) Registry report indicates that approximately half of pediatric lung transplant recipients receive some form of induction.
Consensus has not been reached on whether induction therapy should be used, and centers who use induction therapy have not reached a consensus as to the best agent to use. Most often, the agents used for induction are potent immunosuppressants that may potentially increase the risk for infection or malignancy. In general, induction agents can be divided into lympholytic agents and interleukin-2 (IL-2) receptor antagonists.
Lympholytic agents
Lympholytic agents include rabbit antithymocyte globulin (RATG [Thymoglobulin]), muromonab-CD3 (OKT3 [Orthoclone]), and equine antithymocyte globulin (lymphocyte immune globulin [ATGAM]). These preparations contain antibodies to human lymphocytes and are derived from animal serum. They induce opsonization and phagocytosis of T lymphocytes and modulate T-cell activation.
Lympholytic agents are used by some transplant centers for induction therapy just after removal of the diseased lungs and prior to implantation or immediately after transplantation; they are typically administered for 3-5 days. In other instances, they are used to treat steroid-resistant rejection and are typically administered for 10-14 days.
Results from trials comparing induction agents suggest that patients receiving OKT-3 have a higher incidence of infection. RATG and ATGAM are fairly similar, although RATG infusions may be better tolerated. In a single-center prospective trial, RATG did decrease the incidence of acute rejection. No study has documented that any of these agents have a beneficial impact on the incidence of BO.
Potential adverse effects include the following:
-
Cytokine release syndrome (ie, chills, fever, vomiting, diarrhea, headache)
-
Increased incidence of infection
-
Increased risk of posttransplantation lymphoproliferative disorder ( PTLD)
-
Leukopenia
Alemtuzumab (Campath), a newer monoclonal antibody, binds to the CD52 surface antigen and has been used in adult lung transplant recipients. [34, 35, 36] A systematic review and meta-analysis of observational studies found that alemtuzumab use in heart and lung transplantation was associated with lower rejection rates when compared with conventional induction therapy agents (antithymocyte globulin, basiliximab, and tacrolimus). [37] A retrospective analysis of 5-year outcomes in adult lung transplant recipients found that alemtuzumab induction may be associated with improved outcomes. [38]
IL-2 receptor antagonists
IL-2 receptor antagonists are monoclonal antibodies that specifically bind to the IL-2 receptor on activated T cells. Basiliximab (Simulect) is now the only one available in the United States.
Basiliximab appears to reduce the frequency of acute rejection in adult lung transplant recipients. [39] A retrospective study in pediatric lung transplant recipients found no difference in acute rejection or BO compared with controls. [36] These agents are typically well tolerated.
Maintenance Immunosuppression
Calcineurin inhibitors
Cyclosporine and tacrolimus are the 2 calcineurin inhibitors currently available for use. These powerful agents are the mainstay of immunosuppression and are responsible for the success of transplantation. However, because of an array of potential adverse effects and drug interactions, they have limitations.
Cyclosporine binds cyclophilin in the cytoplasm of the T cell, which then binds calcineurin, a Ca2+ phosphatase, blocking it from activating transcription factors and ultimately preventing the expression of interleukin (IL)-2. IL-2 is a growth factor for T cells. Without it, the magnitude of T-cell response is greatly diminished. Tacrolimus, a macrolide antibiotic, binds a different immunophilin, the FK-binding protein. This complex also binds calcineurin and prevents IL-2 expression. It is more potent than cyclosporine.
Multiple trials have compared the 2 drugs; they are equal in prevention of bronchiolitis obliterans (BO) and promotion of survival. Both drugs are metabolized in the liver by the cytochrome P450 (CYP) 3A4 isoenzyme and can increase the risk of infection, nephrotoxicity, neurotoxicity, gastrointestinal disturbances, electrolyte derangements, malignancy, and hypertension. However, the adverse effect profiles of the 2 agents are clearly different. Cyclosporine may cause gingival hyperplasia and hirsutism, whereas tacrolimus may cause more hyperglycemia.
Most pediatric lung transplant centers now preferentially use tacrolimus-based regimens as their primary immunosuppression because it has a more manageable adverse effect profile in children and because of the potential impact on adherence.
Drug levels must be monitored on a regular basis. Doses for lung transplant recipients are usually maintained at higher trough levels (ie, cyclosporine 300-350 ng/mL; tacrolimus 10-12 ng/mL) than those for other organ recipients. The serum levels require maintenance at levels high enough to prevent rejection without causing debilitating toxicities. Although trough levels are commonly used at most transplant centers, 2-hour levels for cyclosporine and 4-hour levels for tacrolimus are more reflective of the area under the curve for each drug.
Numerous agents may cause changes in the serum levels of cyclosporine and tacrolimus when coadministered. The following are examples of drugs likely to increase cyclosporine or tacrolimus serum levels secondary to decreased hepatic metabolism by inhibiting the CYP3A4 isoenzyme:
-
Cimetidine
-
Erythromycin
-
Clarithromycin
-
Diltiazem
-
Verapamil
-
Itraconazole
-
Voriconazole
-
Fluconazole
-
Ketoconazole
-
Grapefruit juice
The following are examples of drugs likely to decrease cyclosporine or tacrolimus serum levels by either inducing hepatic metabolism via CYP3A4 or decreasing bioavailability of the drugs:
-
Rifampin
-
Phenytoin
-
Carbamazepine
-
Phenobarbital
-
Ethambutol
-
St John’s wort
-
Antacids
Nonsteroidal anti-inflammatory drugs (NSAIDs) can potentiate the nephrotoxicity of these agents and should never be used concurrently. Renal prostacyclin synthesis is important in maintaining glomerular filtration rate and renal blood flow. NSAIDs suppress renal thromboxane-A2 production, which attenuates the effects of reduced vasodilatory prostaglandins. In addition, tacrolimus and cyclosporine should never be used together, as they potentiate their nephrotoxicities.
Initial single-center studies suggest that administraiton of aerosolized cyclosporine may provide a substantial survival advantage to lung transplant recipients. [40] Further studies are necessary.
Cell toxins
Cell toxins used for immunosuppression include azathioprine (Imuran) and mycophenolate mofetil (MMF, Cellcept). Azathioprine prevents the proliferation of T cells by inhibiting purine biosynthesis. Its use is limited because of its toxicity on other cell types. MMF inhibits an enzyme responsible for balancing purine nucleotides in lymphocytes and affects only the de novo pathway of purine biosynthesis. Other cell types are theoretically protected from the full effects of this agent because they can synthesize purine nucleotides via the salvage pathway.
Several studies that compared azathioprine with MMF in lung transplant recipients have not shown a clear clinical benefit of one agent over the other. [41]
Dosing is often determined by white blood cell (WBC) count. The goal is to maintain a total leukocyte count of 4000-7000/µL and an absolute neutrophil count of more than 1500/µL. MMF serum levels can be measured, and data support maintaining levels at approximately 3 mcg/mL.
Potential adverse effects for both of these agents include myelosuppression, infection, and nausea. In addition, azathioprine may cause hepatotoxicity and rash. MMF may cause diarrhea, emesis, and an increased risk of lymphoproliferative disorders. Allopurinol should be used cautiously in patients on azathioprine. The dose of azathioprine should be reduced by 50-75% to minimize the potentiation of toxicity when used in concert with allopurinol.
Corticosteroids
Corticosteroids are the oldest group of immunosuppressant agents. They interfere with several different steps in the inflammatory cascade. They interfere with transcription factors such as nuclear factor kappaB (NF-kB) and activator protein 1 (AP-1), blocking cytokine production. They also cause lymphocyte apoptosis.
Corticosteroids possess many potential adverse effects, including increased risk of infection, hyperglycemia, hypertension, cataract formation, bone loss, gastrointestinal disorders, mood alteration, acne, growth suppression, and amenorrhea. At high doses, corticosteroids may cause alterations in serum levels of the calcineurin inhibitors (cyclosporine and tacrolimus).
Sirolimus and derivatives
The mammalian target of rapamycin (mTOR) inhibitors include sirolimus (rapamycin; Rapamune) and everolimus (40-0-[2-hydroxyethyl]-rapamycin [RAD]). Although they bind to the FK-binding protein, like tacrolimus, they do not inhibit calcineurin. Their effects occur later in the cell cycle, through inhibition of cytokine-induced signal transduction pathways. Because mTOR inhibitors work by different mechanisms than calcineurin inhibitors, agents from these two families of drugs can be used together.
The addition of sirolimus can permit dose reduction or even discontinuation of calcineurin therapy. Sirolimus may help prevent acute rejection, and may have a beneficial effect in patients with chronic rejection because it inhibits proliferation of endothelial and smooth muscle cells in vitro and appears to inhibit vascular injury in vivo. RAD also inhibits smooth muscle proliferation.
Initially, rapamycin was available only as a suspension, but a tablet form is now available. It is routinely dosed once daily, but evidence indicates that in children more desirable drug levels are maintained with a twice-daily dosing regimen. [42] Dosing is adjusted to achieve serum levels of 8-13 mcg/L.
Sirolimus has no role in the immediate postoperative period because it may interfere with wound healing and cause anastomotic dehiscence. Initiation of sirolimus therapy is typically delayed until at least 3 months after transplantation; however, Wojarski et al reported safe use of sirolimus in sirolimus within the first postoperative month in selected lung transplant recipients after an uncomplicated postoperative course and detailed bronchoscopic assessment. [43]
Sirolimus is also associated with the development of interstitial pneumonitis. In addition, when compared with a group of adult lung transplant recipients receiving tacrolimus, azathioprine, and prednisone, those receiving tacrolimus, sirolimus, and prednisone were at significantly greater risk for developing venous thromboembolism (17.2% compared with 3.2%). [44]
Other potential adverse effects of sirolimus include hyperlipidemia and myelosuppression. Lipid profiles must be regularly monitored.
Complications of Lung Transplantation
Lung transplant complications occur in 3 general phases: immediate (less than 1 month), early (1-3 months), and late (beyond 3 months). Immediately post-transplant, the primary complications are technical surgical issues and problems arising from the condition of the recipient or the allograft at the time of implantation.
The early phase is dominated by issues related to the immune response to the allograft, mechanical complications related to the surgical procedure, and adverse effects of immunosuppression (particularly infection). The latter issues continue during in the late phase and also include chronic complications related to the immune response, such as obliterative bronchiolitis and immunosuppression (ie, malignancy). [45]
Primary graft dysfunction
Acute lung injury of newly transplanted lungs has been called numerous names; however, the term primary graft dysfunction (PGD) was the consensus selection of the International Society of Heart and Lung Transplantation (ISHLT).
PGD is an acute lung injury in the transplanted lung in the immediate postoperative period. It results from numerous donor factors, including the very act of dying, which may cause abrupt changes in blood pressure and the release of inflammatory mediators that potentially damage donor organs. These changes are not well understood.
Ischemia-reperfusion injury with an increase in oxygen free radicals is believed to play a critical role in the pathophysiology of PGD. This results in a proinflammatory condition with inflammatory cell activation, release of cytokines and chemokines, and endothelial cell dysfunction, resulting in a dysregulation of vasoactive mediators and prothrombotic and fibrinolytic factors.
However, in kidney transplantation, evidence suggests a reduced incidence of ischemia-reperfusion injury in living related donation compared with cadaveric donation. Additional factors related to the risk of developing PGD include inherent donor lung characteristics, preservation process, pulmonary ischemia and reperfusion, and, possibly, recipient factors (eg, primary pulmonary hypertension).
PGD occurs within hours and as long as 2 days post transplant; it is characterized by poor oxygenation with pulmonary infiltrates on radiographs. Additional characteristics include pulmonary edema, low lung compliance, and increased pulmonary vascular resistance; the histologic examination may reveal diffuse alveolar disease.
The incidence of PGD varies from 10-50%, depending on the definition. The ISHLT also developed a classification of PGD based on severity using the ratio of arterial oxygen pressure to fraction of inspired oxygen (PaO2/FiO2) and chest radiography findings at different time points within the first 72 hours. [46] Contributing factors that may need to be excluded include the following:
-
Hyperacute rejection
-
Cardiogenic pulmonary edema
-
Pulmonary venous obstruction
-
Pneumonia
-
Possibly, transfusion-related acute lung injury
Based on this new classification, the incidence of severe PGD (PaO2/FiO2< 200 48 h posttransplant) appears to be approximately 15%. More importantly, severe PGD is associated with an increase in both early and late graft failure and mortality.
In general, patients with severe PGD are treated similarly to patients with acute respiratory distress syndrome. In patients with capillary leak associated with PGD, excessive fluid should be avoided while maintaining perfusion and adequate oxygenation to vital organs. Typically, protective ventilator strategies using pressure-controlled ventilation with positive end-expiratory pressure (PEEP) and smaller tidal volumes are used to prevent volutrauma and barotraumas to the newly transplanted lungs.
Because pulmonary vascular resistance is often increased, nitric oxide is frequently used to treat PGD. Nitric oxide may also improve perfusion to areas of lung that are being ventilated. Similarly, prostaglandins may be used; experimental data suggests a beneficial role for exogenous surfactant therapy.
When patients require high concentrations of supplemental oxygen (which may contribute to further formation of oxygen radicals) or when they have ventilatory pressures high enough to yield barotrauma or compromise systemic blood return and pressure, the early use of extracorporeal membrane oxygenation (ECMO) should be initiated to provide rest to the lung.
Airway complications
Airway dehiscence was once a common complication of lung transplantation. With the evolution of surgical techniques, this is now rare. However, the development of airway stenosis and bronchomalacia, probably secondary to ischemia-reperfusion injury, is not unusual. If the narrowing is severe enough to cause compromise, the affected area can often be successfully treated by performing balloon dilatation.
Theoretically, this should be more of a problem in young children; however, one study suggests that the incidence of this complication is the same among young children and older children. [47]
Infection
After transplantation, bacterial, viral, or fungal infections may develop. Clinically, patients with posttransplant infection may present with fever, cough, dyspnea, crackles on auscultation, infiltrates on chest radiographs, decreased lung function, or hypoxia.
The likelihood that particular pathogens are responsible is suggested by how long after transplantation the infections appear. Bacteria are the likely causes of infections that occur immediately after transplantation, whereas opportunistic infections (eg, from Pneumocystis jirovecii or Nocardia species) usually are not observed until after the first month post transplant.
To treat bacterial infections, intravenous antibiotics are started intraoperatively. If cystic fibrosis (CF) or septic lung disease is present, antibiotics are initiated based on the results of the most recent sputum culture. If aseptic lung disease is present, patients are routinely given drugs with broad-spectrum coverage.
Viruses that may cause posttransplant infection include the following:
-
Cytomegalovirus (CMV)
-
Respiratory syncytial virus (RSV)
-
Human metapneumovirus
-
Epstein-Barr virus (EBV)
-
Influenza virus
-
Herpes virus
-
Varicella virus
-
Adenovirus
CMV poses a clinical challenge for the transplant physician. [48] CMV can cause acute life-threatening illness, such as pneumonitis or hepatitis. Before the routine use of prophylaxis, CMV was also linked to the development of bronchiolitis obliterans (BO) in lung transplant recipients. Children who are CMV seronegative at the time of transplantation and who receive organs from a seropositive donor are at the highest risk.
Virtually all centers now administer prophylaxis against CMV, although the ideal regimen is a matter of controversy. [49] A randomized clinical trial of valganciclovir prophylaxis in adult lung transplant recipients found that patients who received 12 months of prophylaxis had a markedly lower rate of CMV disease, compared with those who received 3 months of prophylaxis (4% versus 32%, respectively), without an increase in resistance. [50]
RSV infection may be fatal in as many as 12% of all lung transplant recipients. RSV can also potentially trigger acute rejection. Appropriate management in patients with a lung transplant requires further study. The roles of RSV hyperimmune globulin (RespiGam), ribavirin, and palivizumab (Synagis) in this setting are not well defined. In general, community-acquired respiratory viral infections are associated with the development of BO.
Similarly, adequate therapies for adenoviral pneumonia in transplant recipients are lacking. One series studied patients who received cidofovir (1 mg/kg) 3 days per week for 4 weeks, with probenecid and intravenous hydration. Three of the 4 survived the infection, and none developed nephrotoxicity with this regimen; however, 2 of the survivors developed BO.
All of the authors’ transplant recipients are annually immunized against influenza. Patients who have symptoms suggesting influenza are routinely started on therapy with an influenza antiviral agent.
Fungal infections are an exception to the general rule that opportunistic infections usually are not observed until after the first month post transplant. Chronic airway colonization with fungi such as Aspergillus species is not uncommon in patients with CF. If left untreated, these patients may become critically ill soon after transplant.
Besides lung transplant patients with CF, any immunocompromised lung transplant recipient is at risk for developing fungal infections. Candida albicans and Aspergillus species are the most common fungal organisms isolated in this group. Patients with these infections may present with pneumonia but may also present with mediastinitis, wound infections, or mycotic aneurysms.
A prospective single-institution study by Baker et al found that lung transplant recipients had high rates of invasive fungal infection. First episodes of invasive candidiasis occurred a median of 31 days after transplant, while non-Candida invasive fungal infections occurred a median of 86 days afterward. The majority of invasive fungal infections occurred in the absence of recent antifungal prophylaxis; however, breakthrough infections were observed, most often representing failures of micafungin or and aerosolized amphotericin B lipid complex during the transplant hospitalization. These authors suggest providing systemic antifungal prophylaxis targeting Candida for up to 90 days after transplant and extending mold-active prophylaxis for up to 180 days. [51]
Options for treating fungal infections have rapidly increased over the past 5 years with the emergence of newer azoles and echinocandins. Because many patients already have some degree of renal dysfunction from life-long exposure to aminoglycosides, and because the calcineurin inhibitors are nephrotoxic, amphotericin B and intravenous voriconazole should be used cautiously.
In a randomized trial, voriconazole was shown to be superior to amphotericin in treating invasive Aspergillus infection and has the advantage that of being used orally in stable patients. [52] Although effective, itraconazole has somewhat more unpredictable bioavailability. Posaconazole also has potent activity against Aspergillus infection.
The major drawback for any of the azoles is their interaction with cyclosporine, tacrolimus, and sirolimus. In fact, voriconazole is contraindicated in patients on sirolimus, and large downward dose adjustments of the calcineurin inhibitors are indicated when any of the azoles are instituted. Caspofungin is less likely to impact immunosuppressant levels, but tacrolimus levels should still be monitored because they may be lowered by caspofungin. Because caspofungin’s mechanism of action differs, combination with azole therapy is potentially available.
One study reported superiority of an echinocandin-azole combination compared with intravenous liposomal amphotericin B in 90-day survival and renal function; however, strong evidence for use of combination therapy is still lacking. The liposomal form of amphotericin B is less nephrotoxic than amphotericin B and is still indicated for serious fungal infections. Anidulafungin is a newer echinocandin whose dose does not require adjustment in the setting of hepatic dysfunction.
In stable patients, nebulized liposomal amphotericin B is an option, although good randomized studies are lacking. In the setting of lung transplantation, the nebulized form of the drug is most commonly used for prophylaxis. However, optimal duration for prophylaxis is not yet established.
As noted (see above), opportunistic infections, such as those with P jirovecii, tend to occur a month or longer after transplant. However, P jirovecii is a rare pathogen in lung transplant recipients because patients routinely receive prophylaxis with trimethoprim-sulfamethoxazole starting approximately 1 week after transplantation.
Surgical complications
Chylothorax may occur. Damage to the thoracic duct may occur intraoperatively, resulting in a collection of chyle in the pleural space. Treatment is usually conservative, with a decrease in dietary long-chain fatty acids. Surgical intervention is rarely necessary.
Damage to the phrenic nerve may occur intraoperatively, resulting in diaphragmatic paralysis. The nerve is cut only in rare circumstances, but bruising is not uncommon, in which case nerve function usually returns within 3-6 months. However, in the meantime, patients may experience unilateral paralysis, usually without significant symptoms.
Atrial arrhythmias
Atrial arrhythmias—including atrial flutter, atrial fibrillation, or atrial tachycardia—are common complications after lung transplantation, occurring in up to 20% of cases. The development of atrial arrhythmias is associated with longer hospital stay but not with increased mortality. [53, 54]
Acute rejection
Acute rejection remains a fairly common immunologic complication after lung transplantation, although it is not as common as it once was. The lungs are prone to rejection for various reasons. The lungs have a large endothelial surface. The entire cardiac output perfuses the lungs, thereby exposing them to all of the circulating immune effector cells. In addition, the lung has its own array of immune effector cells. Finally, the lungs are constantly exposed to inhaled antigens.
Patients with clinically acute rejection present in the same manner as patients with infections, with cough, fever, decreased lung function, and radiographic changes. Bronchoscopy with bronchoalveolar lavage and transbronchial biopsy is the only definitive way to differentiate rejection from infection.
Rejection is treated with high-dose methylprednisolone (10 mg/kg IV each day for 3 d at the authors’ center). Repeat biopsy is performed 2-4 weeks after therapy. If no improvement occurs, the patient may be given a second course of high-dose methylprednisolone or may be treated with a lympholytic agent for 10-14 days.
Humoral rejection
Humoral (antibody-mediated) rejection is increasingly being recognized as an immunologic complication of lung transplantation. [55] The advent of better techniques to identify the presence of donor-specific antibody (DSA) has opened a new area of study and controversy.
An association in aggregate between the presence of DSA and the development of BO syndrome (see below) is fairly well established. However, the importance of finding DSA in any single asymptomatic individual is unknown, as is the optimal course of therapy. In addition, the role that antibodies to self or third-party human leukocyte antigen (HLA) may play in lung transplant recipients is also unclear.
Plasmapheresis is first line of therapy for established humoral rejection. Intravenous immunoglobulin (IVIG) is frequently administered in conjunction with plasmapheresis to down-regulate B-cell surface antigens and to block antibody binding. In addition, rituximab may be used for B-cell depletion. Bortezomib, a proteasome inhibitor, may be beneficial in treating humoral rejection by causing apoptosis of the plasma cell. However, randomized controlled trials are lacking. [56]
Bronchiolitis obliterans
BO is an inflammatory injury to the small airways, which is presumed to result from chronic rejection in lung transplantation. BO is a histopathologic diagnosis. Because the process is initially patchy and inhomogeneous, transbronchial biopsy samples often do not suggest the diagnosis, necessitating open lung biopsy. Therefore, a corresponding clinical syndrome, termed BOS, was defined that corresponds to the histopathologic diagnosis. [57]
A patient has BOS when an otherwise unexplained drop in lung function occurs after acute rejection, infection, and airway complications have been ruled out. BOS develops in nearly half of all lung transplant recipients within 5 years following transplantation. Risk factors for BOS include recurrent acute rejection (> 3 episodes) or an episode of severe acute rejection. CMV disease had been associated with the development of BOS in many older studies before the advent of routine CMV prophylaxis.
Other risk factors for BOS include the following:
-
Lymphocytic bronchiolitis [58]
-
Gastroesophageal reflux (GER) [61]
-
Respiratory viral infections [62]
-
Development of anti-HLA antibodies [63]
Clinical features of BOS include deteriorating lung function characterized by irreversible obstruction of the small airways that occurs more than 3 months after transplantation. Patients may report dyspnea and wheezing. Physical examination may reveal distant breath sounds, basilar crackles, or unremarkable findings.
Chest radiography findings may be normal or may reveal hyperinflation. High-resolution CT scanning of the chest may demonstrate bronchiectasis, decreased vascular markings, and airtrapping. Ventilation scan may demonstrate retention of xenon consistent with air trapping. Histologically, BO is characterized by a proliferation of fibroblasts into the airway lumen, ultimately forming intraluminal granulation tissue and total obliteration of the lumen. As noted (see above) it is often difficult to make a pathologic diagnosis with routine surveillance transbronchial biopsy.
Until recently, no therapy had been shown to reverse the condition, although some therapies, including augmentation of immunosuppression, seemed to halt further deterioration of the airway obstruction, at least temporarily. However, emerging evidence suggests that patients with early stages of BOS and GER may dramatically benefit from fundoplication. Azithromycin is an antibiotic with anti-inflammatory effects that, in small case series, has shown promise in treating BOS. Larger randomized trials are needed.
Photopheresis is seemingly one of the more promising approaches to treating patients with posttransplant BO. A retrospective review of 60 adult lung transplant recipients treated with photopheresis demonstrated a slowing in the rate of decline in the forced expiratory volume (FEV1). In addition, FEV1 improved in one fourth of those receiving photopheresis. [64]
Infant lung transplant recipients seem to have significantly less acute rejection and BO than older children. In addition, pretransplant mechanical ventilation does not appear to negatively impact infant outcomes. [65]
Malignancy
The use of immunosuppressive agents places lung transplant recipients are at increased risk for malignancies after transplantation. The most common such malignancy in children is posttransplant lymphoproliferative disease (PTLD). [66] The spectrum of disease severity varies widely, with the most severe being true malignancy.
Primary infection with EBV after transplant is the primary risk factor. Therefore, children, who are more likely than adults to be EBV seronegative at the time of transplant, are at increased risk. Furthermore, pediatric lung transplant recipients are at high risk, with an incidence of as much as 19% in one series.
The first therapeutic step is to decrease immunosuppression. This results in regression of the lesion in about 50% of patients but may also increase the risk for later development of BOS. Other potential therapies are poorly studied: the literature is replete with case reports, but randomized controlled trials comparing interventions are lacking. If reduced immunosuppression is unsuccessful, potential therapies include treatment with anti–B-cell antibodies (eg, rituximab), interferon-alfa with or without IVIG, surgical resection, radiotherapy, and cytotoxic chemotherapy.
Historically, the prognosis for patients with PTLD has been poor. The risk for developing BO after having had PTLD is unclear. The interventions described above may substantially improve outcomes for these patients.
Further Outpatient Care
Routine monitoring
Outpatient care post transplant varies somewhat from center to center. After discharge from St. Louis Children’s Hospital, patients are seen in clinic twice a week and regularly attend pulmonary rehabilitation sessions. To prevent complications, or to identify them early, numerous parameters are monitored on a routine basis. Standard laboratory and imaging studies are performed on a routine basis.
Therapeutic drug monitoring
In addition, therapeutic drug monitoring has become a standard for transplant care. Many of the immunosuppressive medications have a narrow therapeutic index, and patients have variable pharmacokinetics. This appears to be particularly relevant in preventing adverse effects from the agents. Patients are instructed to monitor their lung function, blood pressure, temperature, and weight daily and to call with any changes.
Spirometry and oximetry
Rejection or infection frequently results in loss of lung function. For children old enough to perform spirometry, forced expiratory volume in 1 second (FEV1) may be monitored on a daily basis with a home spirometer in order to detect early changes in lung function. For younger children, home oximetry may be used on a daily basis.
Bronchoscopy
As mentioned above, patients undergo their initial bronchoscopic examination while intubated within 24 hours of transplant. At the authors’ center, the first routine surveillance transbronchial biopsy is performed one week after transplantation. At some centers, this procedure may not be performed until one month after transplantation.
At St. Louis Children’s Hospital, the authors routinely perform surveillance transbronchial biopsy at one week and 1, 2, 3, 6, 9, 12, and 18 months after transplantation. Other centers have different monitoring schedules, including some adult centers that perform biopsies only when clinically indicated. [67] The optimal monitoring system has yet to be clearly elucidated and should be an area of further study.
Outcomes After Lung Transplantation
The 1-year survival rate for pediatric lung transplant recipients is close to 85%. The 3-year survival rate after transplantation is now 65%. Overall survival in children after lung transplantation is 5.4 years for patients who received transplants between January 1990 and June 2015. In those who survive past 1 year after transplant, unadjusted survival is 8.8 years. [1]
A study comparing outcomes in pediatric patients with or without cystic fibrosis (CF) who received lung transplants between 1990 and 2017 reported that in-hospital major complications were more common in CF recipients (57% vs 48%, P = 0.003), but 30-day mortality was higher among non-CF recipients (9% vs 5% CF, P < 0.001). One-, five-, and 10-year mortality rates were 18%, 50%, and 65% for CF recipients, respectively, and 21%, 45%, and 58% for non-CF recipients. [68]
The most common causes of death within the first 30 days after transplantation are graft failure, infection, and hemorrhage. In the later posttransplantation period, bronchiolitis obliterans (BO) and infection are the most common reasons for death. [69]
Approximately 80% of survivors have no physical limitations one year after transplantation, and quality of life (QoL) appears to markedly improve; however, definitive data are lacking. Unfortunately, BO syndrome (BOS) remains an almost inevitable long-term complication.
Survival benefit in cystic fibrosis patients
A study by Liou et al suggested that children with CF derive no survival benefit from lung transplantation. [12] The authors, using proportional hazards modeling, developed a model that they believe predicts survival in children with CF. This model differs from their previous model and stands in stark contrast to the literature, in that they could find no value for forced expiratory volume in 1 second (FEV1) that was predictive of survival. They then used this model to ascribe a hazard ratio to lung transplantation, which they introduced into the model as a covariate.
Unfortunately, the study has notable biases and flaws. First, the model assumes that lungs were allocated to patients with the most time on the list without any intervention in that process. However, because lungs were allocated in the United States purely by time accumulated on the list at the time of this study, healthy patients tended to wait longer for lungs. Wait times for lungs could be as long as 2-3 years; thus, transplant centers routinely listed patients to allow the patient to accrue time on the list.
However, the decision to transplant took into account many different factors; if the patient was considered too healthy for transplant or was not ready for transplant, no transplant occurred simply because a set number of days had been accumulated on the list. As the authors denote in their manuscript, this would bias the results and make lung transplant outcomes appear worse.
In addition, the authors used prelisting data to develop their model. These data may have been 3.5 years old or older by the time the patient received a transplant, and their relevance is certainly questionable. This may explain why the model only found that the presence of chronic airway infection with Staphylococcus aureus or Burkholderia cepacia, increasing age, and diabetes were predictive of survival.
Importantly, the authors may have underestimated survival benefit after transplantation because they treated retransplantation as a censoring event. Although retransplantation is indicative of graft loss, it is by no means indicative of death. This may also explain why the authors found a 5-year survival rate of only 34% compared with published data that suggested a 5-year survival rate of 50%.
The authors concluded that only 5 of 514 patients listed for lung transplantation would have derived a survival benefit, despite the fact that 141 of those patients died on the waiting list. In addition, their model indicates that only 1 of 248 patients derived a survival benefit, despite the fact that roughly 90 of those patients were alive 5 years after transplantation. Considering the limitations the investigators faced in designing the model, the findings in this study appear to have been overstated.
Clearly, even a 5-year survival rate of 50% is not satisfactory, and much work is yet to be done to improve outcomes, including improving the selection process to ensure that children likely to benefit from the procedure are the ones who receive a transplant.
Another study reported a breakthrough in transplantation science. Investigators had been hampered by the lack of an adequate animal model to study many of the complications patients routinely face. Okazaki and colleagues at Washington University in St. Louis have developed a technique to perform murine lung transplantation. [70] This new model may provide investigators a new path to study perplexing problems such as BO and primary graft dysfunction (PGD).
-
Number of pediatric lung transplants performed per year as reported to the International Society of Heart and Lung Transplantation (ISHLT) registry.
-
Pediatric lung transplantation actuarial survival by era.
Tables
What would you like to print?
- Overview of Pediatric Lung Transplantation
- History of Lung Transplantation
- Frequency of Pediatric Lung Transplantation
- Indications for Lung Transplantation
- Contraindications to Lung Transplantation
- Clinical Presentation of Pediatric Candidates
- Workup of Pediatric Candidates
- Preoperative Details
- Intraoperative Details
- Postoperative Details
- Induction Regimens
- Maintenance Immunosuppression
- Complications of Lung Transplantation
- Further Outpatient Care
- Outcomes After Lung Transplantation
- Show All
- Media Gallery
- References