Overview
Background
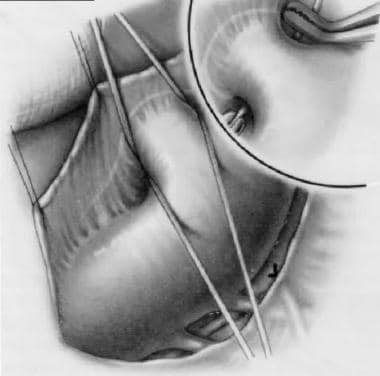
Patent ductus arteriosus (PDA) is a persistent communication between the descending thoracic aorta and the pulmonary artery that results from failure of normal physiologic closure of the fetal ductus (see the image below). In most individuals, the ductus arteriosus is located on the left side; however, if a right aortic arch is present, the ductus arteriosus may be located on the right or left side. The ductus arteriosus is very rarely bilateral.
In normal fetal circulation, the unexpanded lungs receive only 5-8% of the blood entering the pulmonary artery. The ductus arteriosus serves as the predominant route of circulation passing through the right ventricle and pulmonary artery. Approximately 55-60% of the systemic circulation passes from right to left through the ductus.
In the fetus, the oxygen tension is relatively low because the pulmonary system is nonfunctional. This, coupled with high levels of circulating prostaglandins, acts to keep the ductus open. The high levels of prostaglandins result from the little amount of pulmonary circulation and the high levels of production in the placenta.
At birth, the placenta is removed, eliminating a major source of prostaglandin production, and the lungs expand, activating the organ in which most prostaglandins are metabolized. In addition, with the onset of normal respiration, oxygen tension in the blood markedly increases. Pulmonary vascular resistance decreases with this activity. These events result in contraction of the smooth muscle within the wall of the ductus, which results in its closure.
A preferential shift of blood flow occurs; the blood moves away from the ductus and directly from the right ventricle into the lungs. Until functional closure is complete and pulmonary vascular resistance is lower than systemic vascular resistance, some residual left-to-right flow occurs from the aorta through the ductus and into the pulmonary arteries.
In normal-birth-weight and full-term neonates, the ductus arteriosus closes within 3 days after birth. However, the ductus arteriosus is patent for more than 3 days after birth in 80% of preterm neonates weighing less than 750 g, and its persistent patency is associated with increased morbidity and mortality. Furthermore, in the presence of a significant left-to-right ductal shunt in low-birth-weight neonates, a decreased peripheral perfusion and oxygen delivery occurs.
Although PDA is frequently diagnosed in infants, it may not be discovered until childhood or even adulthood. In isolated PDA, signs and symptoms are consistent with left-to-right shunting. The shunt volume is determined by the size of the open communication and the pulmonary vascular resistance. PDA may exist with other cardiac anomalies, which must be considered at the time of diagnosis. In many cases, the diagnosis and treatment of a PDA is critical for survival in neonates with severe obstructive lesions to either the right or left side of the heart.
Surgery is the mainstay of treatment for PDA that does not close spontaneously or with pharmacologic therapy. [1] Two forms of surgical therapy are performed: the traditional surgical approach, which entails a thoracotomy (or alternatively, thoracoscopy), and catheter closure.
Transcatheter closure of PDA has become increasingly popular due to the development of more sophisticated catheter methodologies and devices. [2, 3, 4] Smaller catheters have improved outcomes in preterm infants, a population previously excluded from the procedure. [2, 3] The traditional surgical approach is used when a catheter approach is not possible.
Pharmacologic therapy options in the closure of a PDA include intravenous (IV) indomethacin or ibuprofen and, more recently, antenatal corticosteroids and exogenous surfactant. [5] Preterm infants who are small for gestational age are more likely to require PDA surgery following pharmacologic treatment. [6]
Indications
In infancy, congestive heart failure is an indication for closure of the PDA. If medical therapy is ineffective, urgent intervention to close the structure should be undertaken. Repair may be delayed in the patient who is asymptomatic or well controlled on medical therapy. All PDAs should be closed because of the risk of bacterial endocarditis associated with the open structure. Over time, the increased pulmonary blood flow precipitates pulmonary vascular obstructive disease, which is ultimately fatal.
If an infant has failed to thrive or has overt congestive heart failure, the ductus should be interrupted, regardless of age and size. If the patient is asymptomatic, elective ligation and division can be carried out at approximately age 4 years when the risks of intubation are decreased and the child is more capable of understanding the procedure and process. Some authorities recommend closure any time after age 12 months or when the patient becomes symptomatic.
Contraindications
Relatively few contraindications to closure of a PDA are recognized. The primary contraindication to repair is severe pulmonary vascular disease. If transient intraoperative occlusion of the PDA does not decrease elevated pulmonary arterial pressures with a subsequent increase in aortic pressure, then the closure must be undertaken carefully and may be contraindicated. Closure of the ductus does not reverse preexisting pulmonary vascular disease.
A subset of associated cardiac anomalies—so-called ductal-dependent lesions—depend on flow through the PDA to maintain systemic blood flow. Premature closure of the ductus without concurrent repair of the following defects is contraindicated and may be fatal:
-
Pulmonary artery hypoplasia
-
Pulmonary atresia
-
Tricuspid atresia
-
Transposition of the great arteries
-
Aortic valve atresia
-
Mitral valve atresia with hypoplastic left ventricle
-
Severe coarctation of the aorta
In these patients, all attempts should be made to preserve ductal flow until a more permanent palliative shunt can be constructed or definitive repair can be undertaken.
Contraindications to catheter closure currently involve the size of the patient. Very small premature infants still require surgical closure. Contraindications to surgical closure include concurrent uncontrolled sepsis and an inability of the patient to tolerate general anesthesia.
Outcomes
The prognosis is generally considered excellent in patients in whom the PDA is the only problem. The mortality from surgical closure is less than 0.5%. No deaths have been reported from catheter closure; however, patients undergoing this procedure are part of a select group and have a generally good prognosis otherwise.
If additional cardiac anomalies are present, the risk increases. A high risk is reported in the presence of associated lesions, increased pulmonary vascular resistance, or when the ductus is calcified or aneurysmal.
In premature infants who have other sequelae of prematurity, these sequelae tend to dictate prognosis of PDA. Studies have shown that preterm babies with a gestational age of 30 weeks or younger had a 72% rate of spontaneous closure of PDA. In addition, 28% of children with PDA who were conservatively treated (with prophylactic ibuprofen) reported a 94% closure rate. This rate compared well with rates reported in literature following medical treatment (80-92%). Surgical mortality in premature infants ranges from 20% to 41%.
In the adult patient, prognosis is more dependent on the condition of the pulmonary vasculature and the status of the myocardium if congestive cardiomyopathy was present before ductal closure. Patients with minimal or reactive pulmonary hypertension and limited myocardial changes may have a normal life expectancy.
Periprocedural Care
Preprocedural evaluation
Whichever mode of surgical therapy is used (ie, operative or catheter-based), the appropriate preoperative diagnostic testing is obtained to define other coexisting congenital heart lesions. If the catheter-based method is contemplated, the child is hydrated before the procedure to minimize the potential for dye-induced renal insufficiency. If the traditional surgical approach is used, appropriate preoperative laboratory studies are obtained to assess bleeding tendencies, concurrent infections, or pneumonia in the child.
Radiography
Radiographic findings vary with the size of the ductus and the amount of left-to-right shunting. The earliest findings usually include a prominent main pulmonary artery. With a moderate-sized PDA, the heart shadow is slightly enlarged. A double density of the left atrium may be noticeable. Prominent pulmonary vascular markings or pulmonary edema may be a finding in patients with a large amount of ductal flow.
In patients with a large PDA, findings include marked cardiomegaly with predominant left atrial and left ventricular enlargement, marked enlargement of the main pulmonary artery, and accentuated peripheral pulmonary vascular markings; a prominent ascending aorta; increased pulmonary venous markings, interstitial edema, and pulmonary edema when left ventricular failure intervenes; and possible PDA calcification in adults.
In patients with a moderate-sized PDA, findings include moderate cardiomegaly with prominence of the left ventricle and signs of left atrial enlargement; a prominent main pulmonary artery and increased pulmonary vascular markings in the peripheral lung fields; a prominent ascending aorta; and possible PDA calcification in adults.
In patients with a small PDA, findings are usually normal; possibly, there will be a slight prominence of the main and peripheral pulmonary arteries.
Studies have shown that chest radiographs have limited predictive value in determining which infants will benefit from ligation.
Echocardiography
The aortic end of the PDA can be depicted clearly with 2-dimensional echocardiography. This end is localized first, and the ductus is then tracked back to the pulmonary artery. Precisely documenting the size, shape, and course of the ductus is difficult. High-velocity jets of turbulent flow in the pulmonary artery can be reliably detected with color-flow Doppler imaging. This technique is sensitive in detecting even the smallest PDAs. Echocardiography provides important diagnostic information regarding associated congenital cardiovascular malformations.
Electrocardiography
In patients with a large PDA, electrocardiographic (ECG) findings include left ventricular hypertrophy and left atrial enlargement. When pulmonary hypertension dominates, combined ventricular hypertrophy is noted. When pulmonary vascular disease dominates, there may be evidence only of right ventricular hypertrophy. Patients with a moderate-sized PDA usually have left ventricular hypertrophy; those with a small PDA typically have normal ECG findings.
Cardiac catheterization and angiography
The diagnosis of PDA is almost always based on careful clinical evaluation, including physical examination, chest radiography, echocardiography, and ECG. Right and left heart catheterization may be indicated if other congenital heart lesions are revealed using these methods or if the clinical presentation is unusual.
During the right heart catheterization, the measured oxygen saturation is increased in the pulmonary artery. The shunt and the pulmonary vascular resistance can be calculated to determine the size of the ductus and the presence of pulmonary vascular pathology.
Selective angiography is the definitive tool for determining the presence and size of the ductus. Angiography is also used to define the intracardiac anatomy when other defects are suspected. Color-flow Doppler mapping is more sensitive than cardiac catheterization in detecting a small PDA.
Histology
The walls of the ductus contain intima, media, and adventitia. The medial layer is composed of longitudinal smooth muscle in the inner layer and circumferentially arranged smooth muscle in the outer layers. This is in contrast to true arterial structures, which contain a media primarily composed of circumferential elastic fibers. These layers of smooth muscle contain concentric loose lamina of elastic tissue and a network of tiny thin-walled vessels. The intimal layer is irregularly thickened and contains a considerable amount of mucoid material.
Monitoring and follow-up
Regardless of the method of closure used, patients return for follow-up echocardiography 2-3 weeks after the procedure to assess the closure of the structure. If the traditional surgical approach is used, the patient is also checked for the presence of pulmonary complications (eg, atelectasis, pleural effusion) and for the healing status of the wound.
Technique
Approach considerations
Surgery is the mainstay of treatment for patent ductus arteriosus (PDA). Two forms of surgical therapy are performed: the traditional surgical approach, which entails a thoracotomy, and catheter closure. The traditional surgical approach may involve ligation, division and oversewing, or clip application; the method of closure used depends on the size of the PDA and the experience of the surgeon.
Thoracoscopy remains controversial, with several studies reporting very little benefit in hospital stay or cost compared with the muscle-sparing left lateral thoracotomy approach. Since both techniques are equally acceptable, the choice of surgical technique depends on the surgeon’s preference.
The advent of the catheter-closure technique has changed the policies for ductal management in most institutions. Currently, catheter closure is the accepted treatment for closure of a PDA in most countries in which pediatric cardiac catheterization can be performed.
The efficacy of catheter closure does not equal that of surgical repair. However, with continued improvements, it is likely that these less invasive and less costly techniques will eventually replace surgical treatment in the typical patient.
Operative closure
General approach
General anesthesia is used. Because the ductus is usually located on the left, the patient is positioned for a left thoracotomy (for a ductus on the right, a right thoracotomy is used). In small children, the incision can be limited to several centimeters. The thorax is entered through the third intercostal space in the infant and the fourth intercostal space after infancy.
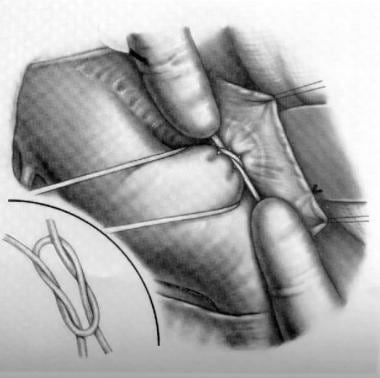
The left superior intercostal vein is identified and ligated. The aorta and main pulmonary artery and the vagus and recurrent laryngeal nerves are identified. Care must also be taken to preserve the thoracic duct. The pericardium is opened, and the PDA is identified, dissected, and ligated (see the image below).
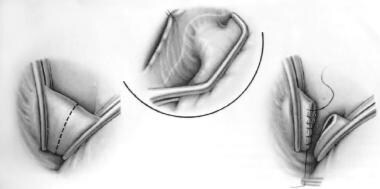
Alternatives to ligation exist for surgical repair of PDAs. The earliest procedures involved double-clamping and dividing the ductus and oversewing each end (see the image below). Although this is still a definitive approach, it is not used nearly as frequently as ligation is, because division is not considered to be an essential part of the closure, and clamping can be risky when the ductal tissue is friable.
A modification of surgical repair, aimed at reducing the amount of dissection around the thin and friable ductus (and thereby reducing the risk of catastrophic bleeding) involves the application of metal surgical clips.
In surgical ligation, the tissue surrounding the ductus must be dissected so that a right-angle clamp can be placed behind it to grasp the silk ligature. This can be hazardous and may result in the tearing of the friable ductus. Once the ductus is torn, the bleeding that ensues may be difficult to control and can be life-threatening. The use of metal surgical clips requires minimal dissection and reduces the risk of this complication.
In addition, surgical ligation takes longer than clip application. Surgical ligation of the ductus necessitates extended retraction of the left lung, thus prolonging the difficulties of ventilation in an already-compromised neonate. An operative technique that requires shorter time is beneficial for sick neonates. Thus, the shorter time for the clip application procedure resulted from the need for less dissection and contributes to fewer perioperative complications.
In adults, cardiopulmonary bypass (CPB) is often necessary to safely close these communications because calcification may preclude ligation and the tissue may fracture, resulting in uncontrolled hemorrhage. A median sternotomy incision provides excellent exposure. The ductus can be patched through an incision in the pulmonary artery under low-flow conditions or after a balloon catheter is passed through the communication to prevent vigorous bleeding from the aorta during closure.
A chest tube is usually placed intraoperatively and removed after 24 hours if no air leak ensues. The typical hospital stay is 3-4 days; however, newer studies report average stays of less than 2 days when a muscle-sparing thoracotomy is performed via an axillary approach or other less invasive incisions.
Thoracoscopy provides an alternative to left posterior thoracotomy. It has been proven useful in both infants and adults. Whether thoracoscopy has benefits over muscle-sparing thoracotomy is debatable. [7] It is not clear if thoracoscopy provides shorter hospital stays or decreases costs. Additionally, thoracoscopy is contraindicated for adults with calcified PDA. Because of the limited control and visualization available, thoracoscopy in neonates is not widely advocated; it appears to have no definite advantage, given that the open procedure uses such a small incision.
Repair in preterm neonates
For neonates weighing 500-1200 g, the procedure is easily accomplished in the neonatal intensive care unit (ICU) under simulated operating room (OR) conditions. Operating in the neonatal ICU is important because these neonates are often in unstable, ventilator-dependent conditions that make transportation to the OR more dangerous.
A 1.5-cm incision based posteriorly near the tip of the scapula is made with the child in the right lateral decubitus position. The lung is retracted medially, and the mediastinal pleura over the descending aorta and proximal subclavian artery is incised and retracted medially. This allows good visualization of the ductus and all important associated structures.
Vascular forceps may, on rare occasions, be used to occlude the structure thought to be the ductus if any doubt exists. At this juncture, distal pulse oximetry and systemic pressure measurements can be used to confirm identification of the PDA. Again, this is rarely necessary. Two or more hemostatic clips can then be used to close the duct. Dividing the duct in these critical neonates is usually not advisable.
Overall benefits of early ligation or medical treatment in regard to decreased bronchopulmonary dysplasia (BPD), hospital costs, and mortality rates are unclear. One study of outcomes of ligation in premature infants showed evidence of early benefit. The 30-day and 1-year mortality rates were 4.8% and 12.8%, respectively. However, the rate of mortality due to BPD at 1-year remained significantly high. [8]
The timing of duct closure is also somewhat controversial, but closure is often recommended when indomethacin therapy fails or is contraindicated in patients with subarachnoid hemorrhage or renal failure. Very low-birth-weight infants treated with indomethacin after PDA diagnosis is less effective than early ibuprofen therapy, resulting in a lower incidence of PDA ligation. [9]
Although definitive data are not available, many aggressive neonatologists believe that early ligation of the ductus is an important adjunct in weaning these premature neonates from the ventilator. Early surgical closure in very premature infants at less than 3 weeks of life is associated with a shortened delay of full oral feeding and improved growth when compared with surgical closure at more than 3 weeks of life. [10]
Preterm babies with a larger PDA area and lower birth weights are likely to fail medical treatment and may benefit from earlier surgical closure. [11] There is a correlation between larger PDA size and higher brain natriuretic peptide levels and failure of medical therapy for PDA closure. [12]
Repair in infants
For PDA repair in the infant, either an OR or a portable OR in the neonatal ICU may suffice. The patient is prepared and draped in the right lateral decubitus position with the left arm extended above the head. Normothermia and proper attention to ventilation are imperative, especially in neonatal patients.
The left lung is retracted medially, with care taken not to compromise ventilation or cardiac output any more than necessary. Meticulous dissection is performed as described (see above), with great care taken to identify the subclavian artery, descending aorta, distal arch, and ductus before ligation; catastrophic mistakes are easier to make than an inexperienced surgeon might imagine. The surgeon must make every effort to identify and preserve the left recurrent laryngeal nerve.
Once the PDA is accurately identified, it is isolated and ligated with either silk suture or stainless steel clips, depending on its size. Several ties or clips are used because placing more than one tie greatly reduces the chance of recanalization. Closing the mediastinal pleura is not necessary after most procedures. A chest tube may be placed at the end of the procedure; however, this step can often be safely omitted, depending on the surgeon’s preference.
Repair in adults
Like neonates and infants, many adolescent and adult patients can be approached via a standard left thoracotomy. When the PDA is large, however, simple ligation may carry some risks, including tearing and hemorrhage. In these cases, gentle double-clamping with appropriate vascular clamps, followed by division and oversewing with 4-0 polypropylene suture is indicated. Because the PDA tends to be short and can be calcified at the aortic end, repair in adults may require a median sternotomy and CPB.
After preparation for CPB is complete, the aorta and pulmonary trunk are separated. Single venous cannulation is sufficient. After CPB is established and the heart is emptied, the patient is placed in the moderate Trendelenburg position, and the PDA is manually occluded by compressing the front wall of the left pulmonary artery against it. This maneuver prevents distention of the pulmonary vasculature and right ventricle.
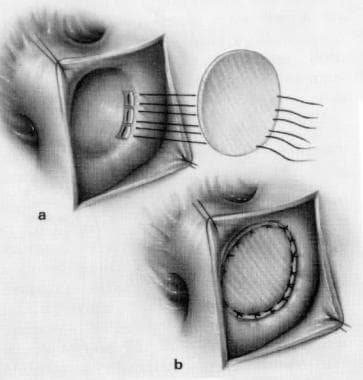
The aorta is cross-clamped, and cardioplegia is infused to arrest the heart. Manual occlusion of the PDA is removed, and the pulmonary artery is opened opposite the PDA. Temporary low-flow perfusion is used while the blood from the duct is blocked with a balloon catheter or finger occlusion. Pledgeted 4-0 or 3-0 polypropylene sutures are used for definitive closure of the ductus. In the rare cases in which the orifice is too large for this closure, a synthetic patch of Dacron or polytetrafluoroethylene (PTFE) can be sewn in (see the image below).
The pulmonary artery is closed with a continuous polypropylene suture, and CPB is terminated in routine fashion after full rewarming. Chest tubes are placed to evacuate air and drain any residual fluid; they may be routinely removed the morning after the operation.
Postoperative care
Surgical repair of the ductus is associated with low, but not absent, morbidity and mortality. After the thoracotomy, the patient usually requires a chest tube for 24 hours and a hospital convalescence of 3-5 days. Once discharged, patients usually require an additional 6-8 weeks of convalescence before returning to unrestricted activity.
Complications of operative closure
Complications of operative PDA closure include bleeding, injury to the recurrent laryngeal nerve, disruption of the thoracic duct with resultant chylothorax, and injury to the vagus nerve. The more serious complications of surgical closure include ligation of the left pulmonary artery, ligation of the descending aorta, or ligation of other arterial structures within the chest; the results of such mishaps are catastrophic. Other developments to watch for include incomplete closure or recanalization, residual shunting, and vocal cord paralysis.
Catheter closure
Transcatheter closure has become a first-line treatment of most PDA in children and adults. Recently, it has become an increasingly attractive option in preterm infants because of advances in device design and delivery options. [2, 3, 4, 13] For catheter closure of the ductus, the patient is prepared and taken to the cardiac catheterization laboratory. In preterm infants the occlusion device is delivered to the ductus through a special 4-11 French delivery catheter via the arterial or venous route. [2, 3] The device is positioned in the ductus, and the closure mechanism delivered.
Postprocedural care
Patients undergoing transcatheter closure of a PDA spend the night following the catheterization in the hospital for observation of the puncture site. They are discharged the following day to full normal activity.
Complications of catheter closure
Complications from transcatheter closure of a PDA include bleeding at the catheterization site, rupture of blood vessels, tachyarrhythmias, bradyarrhythmias, vascular occlusion, inappropriate deployment of the device, migration of the device, and incomplete closure of the ductus. To date, few deaths have been reported, all in very low birth weight infants. [3]
-
Patent Ductus Arteriosus Surgery. Patent ductus arteriosus.
-
Patent Ductus Arteriosus Surgery. Ligation of patent ductus arteriosus.
-
Patent Ductus Arteriosus Surgery. Division and oversewing of patent ductus arteriosus.
-
Patent Ductus Arteriosus Surgery. Patch closure of patent ductus arteriosus.