Background
Aortic valve replacement (AVR) may occasionally be required in infants and children. Common indications for AVR in children include the following:
-
Hypoplasia of the aortic valve annulus in the neonate
-
Progressive stenosis of the aortic valve in infants and children
-
Multilevel left ventricular outflow tract obstruction in association with aortic valve stenosis not amenable to aortic valve repair that requires enlargement of the outflow tract
-
Aortic insufficiency as a complication of percutaneous balloon aortic valvuloplasty
-
Rheumatic aortic valve disease
-
Aortic valve endocarditis
Although no aortic valve substitute is ideal, desirable characteristics include the following:
-
Excellent flow hemodynamics with reduction of left ventricular afterload and/or preload to normal values
-
Lifetime durability without need for reoperation due to structural deterioration
-
Absence of need for anticoagulation
-
Absence of risk for late embolic complications
-
Capability of growth to avoid patient prosthesis mismatch as the child gets older
-
Resistance to infection and endocarditis
-
Ease of implantation
-
Appropriate size availability for all patients
Several aortic valve repair techniques have been used in children, including pericardial leaflet extension, commissural reconstruction, annuloplasty, sinus of Valsalva reduction, sinotubular junction remodeling, and even complete leaflet replacement using autologous pericardium. Aortic valve repair in the child allows for continuing growth and eliminates the need for anticoagulation. However, long-term results have been less than satisfactory, and residual lesions (eg, regurgitation, stenosis) are common.
Despite evidence of stabilization of ventricular dimensions and improvement of functional classification in children following aortic valve repair, the residual lesions continue to progress, with increased regurgitant fraction and/or peak gradients across the left ventricular outflow tract; eventually, patients require reoperation and possible valve replacement. The role of valve repair in children as a curative or a temporizing measure remains incompletely defined. Nevertheless, aortic valve repair delays ultimate replacement until alternative valve replacement options can be offered to patients after completion of somatic growth, pregnancy and increased compliance with anticoagulation regimen.
Mechanical valve prostheses are not ideal valve substitutes in children. Although the incidence of structural valve deterioration is negligible, these prostheses have significant limitations at the time of implant due to the lack of appropriately sized prostheses for small children and neonates. In addition, the absence of potential for growth can result in patient-prosthesis size mismatch as the child grows and may require re-replacement. Moreover, mechanical valves require lifetime anticoagulation with associated activity limitations, difficulties with future pregnancy, and a lifetime risk of thromboembolic and bleeding complications due to potential poor compliance with anticoagulation protocol.
Homografts and bioprosthetic valves are also problematic in children. Although these biologic valves do not require anticoagulation, they do not allow growth, and their durability in the pediatric population is very limited due to the high risk of accelerated structural valve degeneration and early calcification. In addition, the availability of appropriate sized homografts and bioprostheses can be a problem.
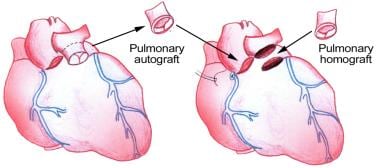
The Ross procedure using pulmonary autograft provides excellent hemodynamics flow characteristics, is capable of growth, and does not require anticoagulation. Despite several shortcomings of the Ross procedure, it has emerged as a popular choice for AVR in infants and children. This review focuses on the role of the Ross procedure in the treatment of aortic valve disease in children and young adults.
History of the Procedure
The procedure of replacing the aortic valve with the patient's own pulmonary valve and then using a pulmonary allograft to replace the pulmonary valve is commonly referred to as the Ross procedure. Lower, Stofer, and Shumway investigated the concept in 1960 using autotransplantation of the pulmonic valve into the descending thoracic aorta of dogs. [1] Pillsbury and Shumway described autotransplantation into the aortic annulus in 1966. [2] However, the first clinical application occurred 1 year later, as reported by Donald Ross in 1967 (see the image below). [3] Since then, the operation has steadily gained acceptance, and the indications for the procedure have expanded.
Relevant Anatomy
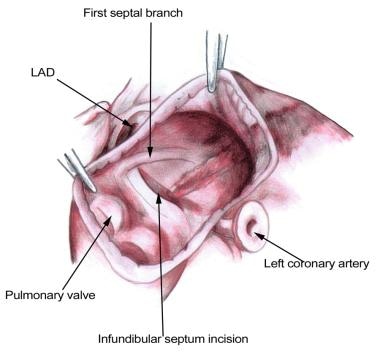
Thorough knowledge of cardiac anatomy is required to perform the Ross procedure. In particular, an understanding the left coronary artery, the first septal branch of the left anterior descending artery, and their relationship to the aortic root and the right ventricular outflow tract is important (see the image below). During harvest of the pulmonary autograft, an appreciation of the subpulmonary conal musculature (ie, the thin muscular tube beneath the pulmonary valve) facilitates the dissection. Knowledge of the configuration of the left ventricular outflow tract and the relationship to the conduction system is important when enlargement of the left ventricular outflow tract is required (Ross-Konno procedure).
Contraindications
The Ross operation is a technically demanding procedure, and the surgeon's experience with this operation and similar procedures affects the decision-making process. Patient factors that affect this process include the patient's age, lifestyle, and coexisting cardiac and noncardiac disease.
Contraindications include the following:
-
Pulmonary valve pathology
-
Known genetic defects in fibrillin, elastin, or collagen in connective tissue disorders (eg, Marfan syndrome, Ehlers-Danlos syndrome)
-
Significant immune complex disease as a coexisting disease, especially if it is the etiology of the aortic valve disease. (eg, systemic lupus erythematosus, ankylosing spondylitis, Reiter disease)
-
Advanced 3-vessel coronary artery disease
-
Significant irreparable mitral valve pathology that requires mechanical valve replacement (considered a relative contraindication by many surgeons)
-
Significant dilatation of the aortic root in comparison to the pulmonary valve annulus associated with aortic regurgitation (considered a relative contraindication by many surgeons, whereas others may continue to offer the Ross procedure along with aortic annular reduction)
-
Pulmonary-valve autograft procedure for aortic valve replacement.
-
Excision of the pulmonary autograft to avoid injury to the underlying first septal branch. LAD = left anterior descending artery.
-
The pulmonary root is dissected out to the bifurcation by taking care to identify the left main coronary artery (LCA). Ao = aorta; PA = pulmonary artery; RCA = right coronary artery; SVC = superior vena cava.
-
The infundibulum is incised 1-1.5 cm proximal to the leaflets of the pulmonary valve.
-
Placement of the pulmonary autograft into the aortic position with polytetrafluoroethylene (Teflon; DuPont, Wilmington, DE) felt reinforcement.
-
Placement of the pulmonary homograft into the pulmonary position.
-
Completed Ross procedure.
-
The left ventricular incision to enlarge the outflow tract during a Ross-Konno procedure. LV = left ventricle; RV = right ventricle.
-
A polyethylene terephthalate (Dacron; DuPont, Wilmington, DE) patch is used to widen the left ventricular outflow tract (LVOT) in the Ross-Konno procedure.