Background
Atrioventricular septal defects (AVSDs) represent approximately 5% of congenital cardiac abnormalities [1, 2] and are bound by a variable deficiency of the atrioventricular (AV) septum immediately above and below the AV valves. These defects are frequently associated with other cardiac malformations. About 30-40% of the cardiac abnormalities observed in patients with Down syndrome are AVSDs. [3]
The AV valves are invariably abnormal in patients with atrioventricular septal defects. At one end of the spectrum of atrioventricular septal defects, incomplete atrioventricular septal defects, also termed ostium primum atrial septal defects (ASDs), have only a deficiency in the inferior portion of the atrial septum immediately superior to the AV valves and have 2 valve orifices. The other end of the spectrum encompasses complete atrioventricular septal defects, with both ASDs and ventricular septal defects (VSDs) and a single common AV valve.
In addition to the term "atrioventricular septal defects," these congenital abnormalities have been described by several other terms, including AV canal defects, endocardial cushion defects, and AV communis.
History of the Procedure
In 1955, Lillehei and colleagues reported the first successful repair of an atrioventricular (AV) septal defect (AVSD) using the technique of cross circulation. [4] Early mortality rates for the repair of AVSDs were 50%. Complications, including complete heart block and AV valve regurgitation, were also common.
In 1958, Lev delineated the bundle of His, which helped decrease the incidence of heart block following surgery. [5] An improved understanding of the structure and function of the common AV valve and a realization of the importance of closing the mitral cleft led to refinements in surgical technique that have decreased the short-term and long-term incidence of AV valve regurgitation. By the 1970s, improvements in surgical techniques and cardiopulmonary bypass resulted in the ability to repair AVSDs with low morbidity and mortality rates in children. Further refinements have allowed successful repair of even complex variations of AVSDs in infancy.
Problem
Atrioventricular septal defects (AVSDs) represent a spectrum of defects involving varying degrees of deficiency of the atrial and ventricular septae. The common pathophysiology is right-to-left shunting at the atrial level, ventricular level, or both. These septal defects are accompanied by atrioventricular (AV) valve abnormalities, which may lead to regurgitation, further complicating the problem. The goals of surgical treatment are to close the atrial and ventricular defects while preserving or improving AV valve function in both the short term and the long term.
Pathophysiology
The embryologic abnormality in atrioventricular (AV) septal defects (AVSDs) is failure of the proper development of the endocardial cushions, which are responsible for the septation of the atria and ventricles. The exact causal factors are unknown.
In the absence of left AV valve regurgitation, the hemodynamic features are the result of left-to-right shunting at the atrial and ventricular levels. In the absence of ventricular level shunting, the hemodynamics resemble those of a typical secundum atrial septal defect (ASD) with right atrial (RA) and right ventricular (RV) volume overload. As with an uncomplicated ASD, the natural history of decades of chronic volume overload results in atrial dilatation and arrhythmias, ventricular dysfunction, and, potentially, pulmonary vascular disease.
Incomplete AVSD
Moderate or severe left AV valve regurgitation occurs in approximately 10% of patients with an incomplete atrioventricular septal defects. The regurgitant jet is often directed into the RA and is often termed a left ventricle (LV)–to–RA shunt. Although this term is not strictly accurate because the jet actually goes from the LV to the left atrium (LA) to the RA, the result is an increase in the magnitude of the left-to-right shunt.
Complete AVSD
Patients with a complete AVSD with both atrial and ventricular level shunting usually present early in infancy with signs and symptoms of congestive heart failure (CHF). In addition, moderate or severe left AV valve regurgitation occurs in approximately 10% of patients with a complete AVSD, worsening the clinical picture. According to Newfeld et al, as many as 90% of untreated individuals with a complete AVSD develop pulmonary vascular disease by age 1 year because of the large left-to-right shunt, potentially exacerbated by associated AV valve regurgitation. [6]
Epidemiology
Presentation
The clinical presentation of a patient with an incomplete atrioventricular (AV) septal defect (AVSD) with isolated atrial level shunting is similar to that observed in a patient with a typical secundum atrial septal defect (ASD). Upon physical examination, an active precordium, a pulmonary outflow murmur, and a fixed widely split second heart sound is present.
Incomplete AVSD
Clinical presentation is complicated by a moderate or severe left AV valve regurgitation in approximately 10% of patients with an incomplete AVSD. The regurgitant jet is often directed into the right atrium (RA) and is often termed a left ventricular (LV)-to-RA shunt (although, more accurately, it is termed an LV-to-left atrium [LA]-to-RA shunt) and increases the magnitude of the left-to-right shunt. These patients may present early in life with symptoms of congestive heart failure (CHF), including pulmonary congestion and infection, dyspnea, tachycardia, and failure to thrive. Inability to medically control the CHF is an indication for earlier surgical intervention in these patients. However, symptoms in the first year of life may indicate the presence of associated left-sided anomalies, which exacerbate the left-to-right shunt and include LV hypoplasia, LV outflow tract obstruction, and aortic arch obstruction.
Complete AVSD
Patients with a complete AVSD with both atrial and ventricular level shunting usually present early in infancy with signs and symptoms of CHF. Clinical presentation is worsened by moderate or severe left AV valve regurgitation, which occurs in approximately 10% of patients with a complete AVSD. Upon physical examination, the precordium is hyperactive, often with a prominent thrill. Auscultatory findings include a systolic murmur along the left sternal border, a high-pitched murmur at the apex resulting from left AV valve regurgitation, and a mid-diastolic flow murmur across the common AV valve. In the presence of elevated pulmonary vascular resistance, a split first heart sound may be present.
Indications
Partial atrioventricular (AV) septal defect (AVSD)
For a partial AVSD, also termed a primum atrial septal defect (ASD), the hemodynamics resemble that of a typical secundum ASD with right atrial (RA) and right ventricular (RV) volume overload. As with an uncomplicated ASD, the natural history of decades of chronic volume overload results in atrial dilatation and arrhythmias, ventricular dysfunction, and, potentially, pulmonary vascular disease. Therefore, repair is indicated and is usually performed by age 2-4 years in a patient with partial AVSD.
Incomplete AVSD
Moderate or severe left AV valve regurgitation occurs in approximately 10% of patients with an incomplete AVSD. The regurgitant jet is often directed into the RA, resulting in an increase in the magnitude of the left-to-right shunt. These patients may present early in life with symptoms of congestive heart failure (CHF), including pulmonary congestion and infection, dyspnea, tachycardia, and failure to thrive. Inability to medically control CHF is an indication for earlier surgical intervention in these patients.
Complete AVSD
According to Newfeld et al, as many as 90% of untreated individuals with a complete AVSD develop pulmonary vascular disease by age 1 year as a result of the large left-to-right shunt, potentially exacerbated by the associated AV valve regurgitation. [6] Patients with trisomy 21 tend to develop pulmonary vascular obstructive disease earlier than infants with normal karyotypes because of small airway disease, chronic hypoventilation, and elevated partial pressure of carbon dioxide (PCO2). Undertake initial aggressive medical management to relieve symptoms of CHF. Perform elective surgical correction by age 3-6 months in infants with AVSD. Earlier intervention is indicated for failure of medical management.
Relevant Anatomy
From a surgical standpoint, the most useful classification subdivides atrioventricular (AV) septal defects (AVSDs) into incomplete and complete, based on AV valve morphology.
Incomplete AVSD
Incomplete or partial defects have 2 AV valve orifices as a result of the continuity between the left superior leaflet (LSL) and the left inferior leaflet (LIL). Although the development of the commissures varies, 6 leaflets are usually present (see the image below).
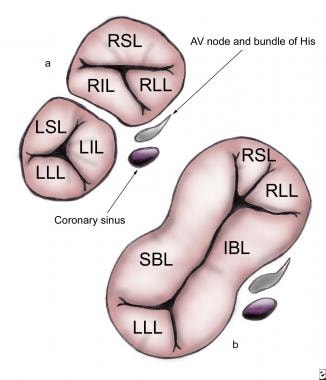 (a) An incomplete atrioventricular septal defect (AVSD) with right superior leaflet (RSL), right lateral leaflet (RLL), right inferior leaflet (RIL), left superior leaflet (LSL), left lateral leaflet (LLL), and left inferior leaflet (LIL). (b) A complete ASD with superior bridging leaflet (SBL), inferior bridging leaflet (IBL), LLL, RSL, and RIL. The locations of the atrioventricular (AV) node and bundle of His are indicated. All images are surgeon's-eye views with cranial leftward, caudad rightward, superior upward, and posterior downward.
(a) An incomplete atrioventricular septal defect (AVSD) with right superior leaflet (RSL), right lateral leaflet (RLL), right inferior leaflet (RIL), left superior leaflet (LSL), left lateral leaflet (LLL), and left inferior leaflet (LIL). (b) A complete ASD with superior bridging leaflet (SBL), inferior bridging leaflet (IBL), LLL, RSL, and RIL. The locations of the atrioventricular (AV) node and bundle of His are indicated. All images are surgeon's-eye views with cranial leftward, caudad rightward, superior upward, and posterior downward.
The right-sided AV valve consists of the right superior leaflet (RSL), right lateral leaflet (RLL), and right inferior leaflet (RIL). The LSL, left lateral leaflet (LLL), and LIL form the left-sided AV valve. The commissure between the LSL and LIL represents the cleft of the left AV valve.
Although most incomplete AVSDs have no ventricular level shunting, classification of an AVSD as complete or incomplete depends only on the valve anatomy and not on the presence or absence of a ventricular septal defect (VSD). As in all AVSDs, the inlet septum in incomplete AVSDs is deficient. The continuity of the LSL and LIL forms a bridge of tissue, which obliterates the potential for shunting below the leaflets. Incomplete defects without associated ventricular level shunting have also been termed ostium primum atrial septal defects (ASDs), whereas those with a VSD have been described as intermediate or transitional AVSDs.
Complete AVSD
Complete AVSDs have a single common AV valve orifice resulting in a 5-leaflet valve (see the image below).
 (a) An incomplete atrioventricular septal defect (AVSD) with right superior leaflet (RSL), right lateral leaflet (RLL), right inferior leaflet (RIL), left superior leaflet (LSL), left lateral leaflet (LLL), and left inferior leaflet (LIL). (b) A complete ASD with superior bridging leaflet (SBL), inferior bridging leaflet (IBL), LLL, RSL, and RIL. The locations of the atrioventricular (AV) node and bundle of His are indicated. All images are surgeon's-eye views with cranial leftward, caudad rightward, superior upward, and posterior downward.
(a) An incomplete atrioventricular septal defect (AVSD) with right superior leaflet (RSL), right lateral leaflet (RLL), right inferior leaflet (RIL), left superior leaflet (LSL), left lateral leaflet (LLL), and left inferior leaflet (LIL). (b) A complete ASD with superior bridging leaflet (SBL), inferior bridging leaflet (IBL), LLL, RSL, and RIL. The locations of the atrioventricular (AV) node and bundle of His are indicated. All images are surgeon's-eye views with cranial leftward, caudad rightward, superior upward, and posterior downward.
These leaflets are termed the left superior or superior bridging leaflet (SBL), left inferior or inferior bridging leaflet (IBL), LLL, RSL, and RIL. Alternatively, the superior and inferior leaflets also may be termed anterior and posterior, respectively.
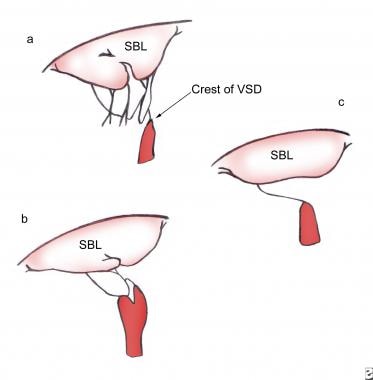
Rastelli et al further subclassified complete AVSDs into types A, B, and C based on the morphology of the SBL (see the image below). [7]
In a Rastelli type A defect, the SBL is divided at the plane of the interventricular septum and attached to the crest of the VSD by numerous cordae. Type B complete AVSDs, which are rare, are characterized by cordal attachments from the left AV valve to papillary muscles in the right ventricle (RV). In a Rastelli type C defect, the SBL is said to be "free floating" because it is undivided and unattached to the crest of the VSD.
Aortic valve displacement
The aortic valve is displaced anterosuperiorly and to the right. As mentioned above, in all forms of AVSDs, the inlet portion of the left ventricle (LV) is deficient relative to the outflow tract. This decrease in the inlet-outlet ratio results in the characteristic gooseneck deformity observed on anteroposterior projection of a left ventriculogram. The LV outflow tract is elongated and horizontally oriented. According to Studer et al and Piccoli et al, although frequently narrow, the LV outflow tract causes obstruction in only 4-7% of individuals with AVSDs. [8, 9]
Ventricular balance
Both left and right AV valves may equally share the common AV valve orifice. This arrangement is termed a balanced defect. Occasionally, the orifice may favor the right AV valve (right dominance) or the left AV valve (left dominance). In marked right dominance, the left AV valve and LV are hypoplastic; frequently, they coexist with other left-sided abnormalities including aortic stenosis, hypoplasia of the aorta, and coarctation. Conversely, marked left dominance results in a deficient right AV valve with associated hypoplasia of the RV, pulmonary stenosis or atresia, and tetralogy of Fallot (TOF). Ventricular balance is based on the size of the ventricular inlet, not on the size of the ventricular chamber, and is assessed best on the 4-chamber view on echocardiography.
Conduction tissue location
Because the conduction tissue is at risk during repair, its location is of importance in the surgical treatment of AVSDs. The AV node is displaced posteriorly and inferiorly toward the coronary sinus in what has been termed the nodal triangle, which is bounded by the coronary sinus, the posterior attachment of the IBL, and the rim of the ASD (see the image below).
The bundle of His courses anteriorly and superiorly to run along the leftward aspect of the crest of the VSD, giving off the left bundle branch and continuing as the right bundle branch.
Other cardiac anomalies
According to Bharati et al, numerous other cardiac anomalies are associated with AVSDs including patent ductus arteriosus (found in 10% of individuals with AVSD) and TOF (found in 10% of individuals with AVSD). [10] Of the important abnormalities of the left AV valve, according to Draulans-Noe et al, 2-6% of patients with AVSD have a single papillary muscle (parachute mitral valve) and 8-14% of persons with AVSD have a double-orifice mitral valve. [11] Bharati et al state that a persistent left superior vena cava, with or without an unroofed coronary sinus, is encountered in 3% of patients with an AVSD. [10] Double-outlet RV, which is found in 2% of individuals with AVSD, significantly complicates or may even preclude complete surgical correction.
As mentioned previously, LV outflow tract obstruction from subaortic stenosis or redundant AV valve tissue occurs in 4-7% of individuals with AVSD, according to Studer et al and Piccoli et al. [8, 9] Associated transposition of the great arteries and LV inflow obstruction have rarely been reported.
Contraindications
The treatment of choice for an incomplete or complete atrioventricular (AV) septal defect (AVSD) is complete surgical repair. Pulmonary artery banding for palliation of symptoms of congestive heart failure (CHF) has a limited role in the management of these lesions. Indications for pulmonary artery banding may include patients with associated complex cardiac anomalies, severely unbalanced defects, or other functional single ventricle anatomy necessitating an ultimate Fontan procedure and a poor clinical condition precluding major cardiac surgery.
According to Newfeld et al, as many as 90% of untreated patients with a complete AVSD develop pulmonary vascular obstructive disease. [6] As with a secundum atrial septal defect (ASD), patients with a partial AVSD are at risk for developing pulmonary vascular obstructive disease in the third, fourth, and fifth decades of life. Cardiac catheterization is recommended for patients presenting later in life for repair. A pulmonary vascular resistance of greater than 10 Wood units is a contraindication to surgical repair.
-
(a) An incomplete atrioventricular septal defect (AVSD) with right superior leaflet (RSL), right lateral leaflet (RLL), right inferior leaflet (RIL), left superior leaflet (LSL), left lateral leaflet (LLL), and left inferior leaflet (LIL). (b) A complete ASD with superior bridging leaflet (SBL), inferior bridging leaflet (IBL), LLL, RSL, and RIL. The locations of the atrioventricular (AV) node and bundle of His are indicated. All images are surgeon's-eye views with cranial leftward, caudad rightward, superior upward, and posterior downward.
-
Rastelli classification. (a) Rastelli type A. (b) Rastelli type B. (c) Rastelli type C.
-
The common atrioventricular (AV) valve is floated to a closed position using isotonic sodium chloride solution. The central apposition points of the superior and inferior bridging leaflets are identified and marked with fine polypropylene sutures.
-
Two-patch technique. A patch of polytetrafluoroethylene (Gore-Tex) is fashioned and secured along the crest of the ventricular septal defect.
-
Two-patch technique. Interrupted horizontal mattress sutures are placed through the crest of the ventricular septal defect (VSD) patch and the inferior and superior bridging leaflets, dividing the common atrioventricular (AV) valve into right and left components.
-
Two-patch technique. The pericardial patch is secured to the crest of the prosthetic ventricular septum with the superior and inferior bridging leaflet sandwiched between the 2 patches.
-
One-patch technique. The superior and inferior bridging leaflets are divided into right and left components.
-
One-patch technique. The leaflets are resuspended to the patch by passing sutures through the cut edge of the left atrioventricular (AV) valve leaflet, the patch, and the cut edge of the right AV valve and tying the sutures.
-
The cleft of the mitral valve between the superior and inferior bridging leaflets is closed.
-
The atrial septal defect (ASD) is closed with an autologous pericardial patch. The coronary sinus is placed in the left atrium to avoid injury to the conduction system. The rim of the ASD, the atrioventricular (AV) node, and the bundle of His are indicated. The dashes represent the proposed suture line.