Effects of Altitude
Studies of the effects of chronic hypoxemia can be performed in the laboratory by decreasing either the concentration of inspired oxygen or the barometric pressure in a hypobaric chamber. [1] Nature has provided a third option, high altitude, which allows for the examination of the effects of chronic hypoxemia in individuals under varying conditions. [1]
Thus, studies of high-altitude physiologists are of interest not only to mountaineers and aviators but also to physicians. Knowledge gained on mountain peaks may give insight into the pathophysiology of patients with cyanotic heart disease and chronic lung disease. Finally, physicians caring for patients who already have hypoxemia should understand the alterations provoked by changes in altitude that may affect these patients while they are living in or visiting mountainous regions or traveling by air.
High altitude has generally been defined as an elevation above 2,500-3,000 m (approximately 8,200-10,000 ft). [2] In healthy persons, clinically significant changes are difficult to demonstrate at elevations lower than this. Many people live at high altitude and perform normal activities. Worldwide, more than 140 million people live more than 2,500 m above sea level; of these, 80 million live in Asia, and 35 million live in the Andean mountains (where the major population density is at elevations exceeding 3,500 m). [3]
Mountaineers and aviators have experimented with humans’ ability to function and survive at extreme altitudes. The conquests of Mount Everest (8,884 m [about 29,140 ft]) without supplemental oxygen were a stringent test of survival ability in a severely hypoxemic environment. At that altitude, nearly all of the available oxygen is required to support basal metabolism, and the climbing rate near the summit drops to 2 m/min.
Changes that are seen in healthy individuals at high or extreme altitude may be exaggerated in patients with chronic cardiopulmonary disease; changes may occur at modest elevations. The cardiovascular changes at high altitude are influenced by factors such as population ancestry and sociocultural determinants, as well as adaptation, nutrition, intercurrent infection, exposure to pollutants and toxins, socioeconomic status, and access to medical care. [4, 5, 6]
Barometric pressure
From sea level to high altitude, the percentage of oxygen remains constant at 20.93%; therefore, barometric pressure determines the partial pressure of oxygen (PO2) in ambient air. Barometric pressure decreases as one rises in altitude and moves toward the poles. The changing position of the sun in relation to the equator affects barometric pressure, producing a seasonal atmospheric tide.
At sea level (barometric pressure, 760 mm Hg), the PO2 of ambient air is 159 mm Hg (ie, 760 mm Hg × 0.2093). As air passes through the respiratory tract, it is saturated with water vapor, which makes the inspired PO2 149 mm Hg (ie, [760 – 47 mm Hg] × 0.2093).
The alveolar PO2 (PAO2) is calculated as follows:
PAO2 = [(PB – PH2 O) FiO2] – [PACO2 (FiO2 + 1 – FiO2/R)],
where PB is the ambient barometric pressure, PH2 O is the pressure water vapor exerts at body temperature, FiO2 is the fraction of inspired oxygen, PACO2 is the alveolar partial pressure of carbon dioxide, and R is the respiratory exchange ratio.
Humans have shown an ability to adapt for short periods to a barometric pressure one third that of sea level on Mount Everest without supplemental oxygen. At that elevation, the calculated PAO2 is 35 mm Hg, and the arterial PO2 (PaO2) is 28 mm Hg. Humans can permanently live at 5,100 m (16,700 ft), where the barometric pressure is approximately one half that of sea level. Although cold, low humidity, increased solar radiation, and poor economic conditions limit the ability to survive at high altitude, hypoxia is the most important factor.
Changes in oxygen transport
At sea level, the PO2 available in the atmosphere is adequate to meet the oxygen demands of mitochondria. At each stage of the oxygen transport system, PO2 decreases; this phenomenon is figuratively called the oxygen cascade.
At high altitudes, the decrease in barometric pressure reduces the amount of oxygen initially available in the environment, making the slope of the cascade considerably less steep than it otherwise is. Mechanisms that compensate for the decreased availability of oxygen in the environment include changes in the intracellular enzyme systems to allow them to function at low levels of oxygen and changes in the oxygen transport system to increase the amount of oxygen delivered. The latter is the primary compensatory mechanism. [7]
However, changes occur at all levels of the oxygen transport system, namely, ventilation, pulmonary diffusion, circulation, and tissue diffusion.
A slight increase in ventilation is first noted on ascent to above 1,524 m (5,000 ft). At rest, this increase is manifested primarily as an increased tidal volume. With exercise, both the tidal volume and the respiratory rate increase. Hyperventilation decreases PaCO2 and increases PAO2. This is the most important form of early acclimatization to high altitude. The hypoxia-induced increase in minute ventilation occurs shortly after arrival at altitude and increases during week 1. Minute ventilation decreases slightly later but remains above sea-level values.
An increased hypoxic ventilatory response is an important means of acclimatization for a sea-level resident who aspires to participate in activities at high altitude. Mountain climbers with an increased hypoxic ventilatory response are better able to climb to great heights, presumably because of the increased availability of alveolar oxygen; this capacity may also have a downside. A low hypoxic ventilatory response has been implicated in acute mountain sickness, excessive polycythemia, and low birth weight.
Stimulation of the carotid bodies mediates hyperventilation. With acute exposure, ventilation does not notably increase below 3,000 m (9,840 ft). This situation corresponds to a PAO2 of 60 mm Hg. However, after 4 days of exposure to even modest increases in altitude, ventilation is consistently greater than normal ventilation at sea level.
After a person acclimatizes, hyperventilation may occur at a PaO2 as high as 90 mm Hg. The hypoxic ventilatory response persists for the sea-level resident who continues to remain at high altitude. At extreme altitudes, marked respiratory alkalosis develops to maintain a PAO2 of more than 35 mm Hg. In a decompression chamber with conditions equal to those at Mount Everest, PaCO2 is 8 mm Hg.
In contrast, the native high-altitude resident has a blunted hypoxic ventilatory response (ie, is desensitized) to hypoxia. Improved oxygen usage in the peripheral tissues with decreased ventilatory effort has been postulated as an explanation for this phenomenon. Studies of high-altitude residents showed that for desensitization to occur, exposure to high altitude must occur in early childhood and last for several years. The decrease in hypoxic ventilatory response is first noted after 8 years of age. At the same time, vital capacity increases correspondingly.
After desensitization to hypoxia has occurred in the high-altitude resident, it persists for years, even if the person returns to sea level. Offspring of lowlanders born and raised at high altitude have the same phenomenon as that of native highlanders. The native highlander hyperventilates compared with the lowlander, and the high-altitude resident hypoventilates compared with the newcomer to altitude.
Therefore, native high-altitude residents can perform a given physical activity with a relatively small ventilatory requirement; hence, they have less dyspnea than others do. This advantage increases their capacity to perform work at high altitude.
A patient with cyanotic congenital heart disease also has a blunted hypoxic ventilatory response. This effect develops as early as age 7 or 8 years. The most blunted ventilatory responses have been noted in patients with the highest degree of desaturation. After arterial saturations are brought back to normal by surgical correction and normalized, the ventilatory response to hypoxia returns to normal.
This outcome is unlike that observed in the native highlander, whose response remains blunted for years. An important distinction between the native highlander and the patient with cyanotic congenital heart disease (CHD) is that the highlander has a lowered PAO2, whereas the patient with cyanotic CHD has a normal PAO2, although they both have arterial hypoxemia.
At sea level, the alveolar-arterial (A-a) gradient is 6-17 mm Hg. This gradient may limit exercise by the newcomer to high altitude even if he or she hyperventilates. The development of notable arterial desaturation during exercise suggests this possibility. The native high-altitude resident has a pulmonary diffusion capacity 20%-30% higher than that of a sea-level resident. This capacity helps maximize gas exchange in the alveoli.
Configurational changes of the chest, anatomic changes of the lungs to increase the surface area of the alveoli, and an improved ventilation-perfusion ratio owing to pulmonary hypertension have been offered as possible explanations for this finding. Animal studies in chronically hypoxic newborn rats have shown structural changes that appear to optimize the structure and function of the lungs.
Exposure to high altitude has important implications for the cardiovascular system. On initial ascent, sympathetic activity markedly increases, resulting in an initial increase in heart rate and cardiac output. However, after prolonged exposure, maximal oxygen uptake decreases, stroke volume is lowered, and cardiac output falls below sea-level values. The reduction in stroke volume is thought to be secondary to decreased ventricular filling. Exercise markedly reduces maximum cardiac output; this effect is more pronounced in visitors than in natives.
A 32% decrease in coronary blood flow has been observed after 10 days at 3,100 m (10,200 ft). [8] However, no evidence of myocardial ischemia is observed. This finding is presumably due to increased extraction of oxygen from coronary arterial blood and reduced oxygen requirements secondary to decreased cardiac work. Left ventricular (LV) dysfunction has been suggested; however, echocardiographic indices of LV contractility are normal and chamber sizes are reduced at altitude.
In one study, Bernheim et al found that increased pulmonary arterial pressure occur in association with exercise and altitude hypoxia did not cause LV diastolic dysfunction. [9] The authors concluded, “Ventricular interaction seems not to be of hemodynamic relevance in this setting.” Significant increase in right ventricular (RV) wall thickness and decreased ejection fraction are observed on magnetic resonance imaging (MRI) scans in children with high-altitude pulmonary hypertension. [10]
With increasing hypoxia, the maximum heart rate decreases by 1 beat/min for every 130 m (about 430 ft) above 3,100 m (10,200 ft). The decreased cardiac output, stroke volume, and exercise capacity noted at high altitude may be due to decreased preload secondary to a reduction in plasma volume associated with arrival at high altitude.
Electrocardiographic (ECG) changes after ascent to high altitude have also been described. Right-axis deviation, right precordial T-wave inversion from a normally upright adult T wave, and T-wave changes in the left precordial leads have been described in mountaineers. ECGs of immigrants to high altitude demonstrate an increase in RV hypertrophy with increased duration of high-altitude residence. Loss of normal circadian rhythm and QTc prolongation have been described in both infants and adults.
In general, systemic blood pressure is slightly lower at high altitude than it is at sea level. This difference is thought to be secondary to the vasodilatory effects of hypoxia on the systemic vascular smooth muscle. The incidence of hypertension at high altitude has been reported to be less than that the rate at sea level.
The final step in the oxygen cascade is the diffusion of oxygen from the capillaries to the mitochondria. For understandable reasons, this step has not been extensively studied at high altitude. Increases in the capillary density and decreases in the size of muscle fibers combine to shorten the distance over which oxygen must diffuse. In several species of animals, this response appears to help them adapt to high altitude, but it does not appear in humans until after 40 days of marked hypobaric exposure.
Oxygen-hemoglobin dissociation curve
Tissue PO2 (tPO2) varies little over a PAO2 range of 70-100 mm Hg. As might be expected at high altitude, a PAO2 of 40-70 mm Hg is associated with rapid unloading of oxygen for small changes in oxygen tension. Some suggest that increased oxygen affinity or left-shifting of the oxygen-hemoglobin dissociation curve may be beneficial at high altitude. As with fetal hemoglobin, a leftward shift facilitates oxygen loading in a hypoxic environment. Others suggest that a rightward shift may increase the ability of the blood to unload oxygen at the tissue level.
Studies at 4,520 m (14,830 ft) have demonstrated that the curve shifts to the right under standard laboratory conditions (pH, 7.40; partial pressure of carbon dioxide [PCO2], 40 mm Hg) because of an increase in 2,3-diphosphoglycerate. [11] However, in vivo, the respiratory alkalosis associated with high-altitude hyperventilation results in a leftward shift on the curve. Therefore, the actual PO2 for 50% oxygen saturation (P50) at altitude is not significantly different from that at sea level.
The Mount Everest Medical Expedition revealed a progressive leftward shift at high altitudes as the respiratory alkalosis increased. This effect improves oxygen loading in the lungs.
In summary, at each stage of the oxygen transport system, considerable changes occur to facilitate oxygen delivery. The extent to which the patient with cyanosis makes use of these or similar mechanisms can be a focus of future research.
Hematologic changes
No less important than the transport system is the transport vehicle—namely, the red blood cell (RBC). During the first 1-2 weeks at high altitude, plasma volume decreases, raising the hemoglobin concentration by 1-2 g/dL. In addition, within hours of exposure to altitude, RBC production increases because production of erythropoietin is heightened. However, the overall response is slow, taking months to reach equilibrium.
The degree of polycythemia is directly related to the altitude, up to an elevation of 3,660 m (12,000 ft). Above this altitude, the hemoglobin concentration increases rapidly. However, if the systemic arterial saturation falls below 60%, erythropoietic activity decreases. In subjects living at 4,540 m (14,900 ft), total blood volume gradually rises from 80 to nearly 100 mL/kg, a change that represents an increase in RBC volume as plasma volume decreases. Monge disease (chronic mountain sickness) is associated with excessive erythropoiesis.
Polycythemia is associated with hyperviscosity and declining oxygen transport. A further rise in hemoglobin is observed with age at high altitude. At altitude, climbers with polycythemia exhibit reduced maximal oxygen consumption, even on 100% oxygen. This observation suggests that peripheral extraction of oxygen from blood is limited by its reduced flow. Phlebotomy and hemodilution experiments in mountain climbers and autologous RBC transfusions in athletes have not yielded information about the ideal hematocrit for any given altitude.
The platelet count decreases by 7% after 2 days at 2,990 m (9,800 ft) and by 25% after 2 days at 5,370 m (17,600 ft). Some suggest that exposure to high altitude induces a hypercoagulable state in humans.
Increased fibrinogen levels and a decreased clot lysis time were noted in 38 soldiers living at high altitude for 2 years, as compared with control subjects at sea level. [11] Soldiers with clinical evidence of pulmonary arterial hypertension had somewhat low levels of fibrinogen, high levels of platelet factor III, and increased platelet adhesiveness. This evidence suggests that conversion to fibrin, and possibly platelet deposition, were occurring in these subjects.
Similar studies of the coagulation status of patients with cyanotic congenital heart disease have been conducted. The Operation Everest II project performed in a hypobaric chamber showed no changes in coagulation factors.
Metabolic changes
Most visitors to high altitude notice initial weight loss, which has been attributed to reduced dietary intake, enhanced water loss, and loss of stored body fat. Anorexia is a common complaint of visitors to even moderate altitude. At high altitude, appetite and caloric intake decrease dramatically in unacclimatized persons, who generally find fat distasteful and prefer sweets. Fluid losses result from the insensible water losses associated with hyperventilation and low humidity, as well as diuresis induced by hypoxia and the cold environment.
Changes in sensory, motor, and mental function
Because the retina of the eye has a great requirement for oxygen, vision is the first sense altered with the lack of oxygen. This phenomenon is demonstrated by diminished night vision even at altitudes below 3,000 m (about 9,600 ft). At 3,048 m (10,000 ft), people require more time to learn a new task than they do at low elevations. At 6,100 m (20,000 ft), impairments in sensory, perceptual, and motor performance have been demonstrated. [12]
In acute hypoxia, reduction of arterial oxygen saturation to 85% decreases a person’s capacity for mental concentration and abolishes fine motor coordination. Reduction of saturation to 75% leads to faulty judgment and impaired muscular function.
One year after the American Medical Research Expedition to Everest, reductions in finger-tapping speed persisted. Also observed were declines in visual long-term memory and verbal learning, along with increased aphasic errors during neuropsychological testing after climbs to high altitude. This finding prompted some to surmise whether climbs to extreme altitude cause brain damage.
On initial exposure to altitude, cerebral blood flow (CBF) decreases because of vasoconstriction associated with hypocarbia. However, when PaO2 decreases to 50-60 mm Hg, CBF increases. Blood flow appears to be regionally uneven, increasing at the brainstem level at the expense of cortical flow. This mechanism may possibly explain the increased vulnerability of the cortex to hypoxia.
A surprising observation is that the climbers with a high ventilatory response to hypoxia have the most impairment. The hypocapnia associated with hyperventilation possibly causes a marked decrease in CBF that offsets any beneficial effects of increased oxygenation.
Effects of Altitude on Pulmonary Pressures
Pulmonary arterial pressure is inversely dependent on a person’s age (beyond the neonatal period) and on the environment. At sea level, pulmonary arterial pressure rapidly decreases from the systemic level of the fetus to near-adult levels in the first hours or days after birth. In infants born at high altitude, however, the decrease is both slower and smaller than the decline just described.
In one study, right-heart catheterizations were performed in 32 healthy children aged 1-14 years and living permanently above 4,240 m (14,000 ft). [13] For children aged 1-5 years, the mean pulmonary arterial pressure was 45 mm Hg (normal at sea level, 12-15 mm Hg). It was lower, a mean of 28 mm Hg among children aged 6-14 years.
In anatomic terms, the delayed decrease in the pulmonary arterial pressure is associated with persistence of the fetal elastic fibril pattern and with medial hypertrophy in the pulmonary arterioles. Electrocardiography (ECG) demonstrates persistence of fetal/neonatal right ventricular (RV) dominance.
In Leadville, Colorado (3,100 m [10,200 ft]), mean pulmonary arterial pressure was 25 mm Hg in healthy high school students and increased to 54 mm Hg after exercise. [14] —values surprisingly similar to those in adults during Operation Everest II at a simulated altitude of 8,840 m [29,000 ft] in a hypobaric chamber. Pulmonary arterial pressures at lower altitudes, such as Denver, Colorado (1,610 m [about 5,280 ft]), are near sea-level values. Thus, a critical alveolar oxygen tension (PAO2) appears to mark the level of hypoxia necessary to induce pulmonary vasoconstriction.
A publication from Peru did not report a higher prevalence of pulmonary artery hypertension at an altitude of 4,000 m in the absence of any associated structural heart disease. [15] However, this study reported structural heart defects in 1.5% of the study population (326 children).
Data from newborn calves suggest that pulmonary vascular resistance at a normal pH increases when the partial pressure of oxygen (PO2) falls below 65 mm Hg. [16, 17] These data are supported by the clinical observation in humans that increases in pulmonary arterial pressure are not seen until the PAO2 decreases below 60-65 mm Hg, which corresponds to altitudes higher than 3,000 m (about 9,840 ft).
Noninvasive and invasive methods of evaluating pulmonary arterial pressure were compared in a separate study from Kojonazarov et al. [18] A combination of ECG and Doppler and MRI measurements was found to correlate with cardiac catheterization data. Pulmonary flow acceleration time was found to be a good predictor of pulmonary hypertension.
A study of children in Tibet (3,600-4,600 m [11,811-15,092 ft]) showed significant elevated pulmonary hypertension measured by Doppler echocardiographic studies in children aged 12-24 months. [10]
Among adults from high-altitude areas who move to sea level, pulmonary arterial pressures return to normal in 2 years. In addition, stroke index increases, and heart rate decreases. Inhalation of oxygen at high altitude achieves only modest reductions in pulmonary arterial pressure in comparison with the reductions induced by a permanent change of residence to sea level.
Increased nitric oxide production has also been shown in some studies of European children at high altitude with pulmonary hypertension. [19]
More recent reviews present the state of the art knowledge of responsiveness of pulmonary circulation to acute and chronic hypoxia and current therapeutic options in the management of high-altitude pulmonary hypertension. [1, 20]
Adaptation and Acclimatization
Some residents of newly settled high-altitude communities in the United States may be at increased risk for problems in adapting to high-altitude living. Unlike natives of older communities in the Andes or in Tibet, who have had millennia to evolutionarily adapt to this type of environmental stress, they have not been genetically selected for high-altitude living. [21]
In 1966, Grover et al reported a 15-year-old high-school skiing champion who was living at about 3,000 m (10,000 ft) and was totally asymptomatic. [22] She underwent right-side heart catheterization in Leadville, Colorado, and was found to have pulmonary arterial pressures of 67/27 mm Hg (mean, 44 mm Hg) at rest and 144/85 mm Hg (mean, 109 mm Hg) during exercise. The patient continued to live at high altitude for 2 years and then moved to sea level for 11 months, where she was reexamined. Her pulmonary arterial pressures had fallen to 33/8 mm Hg (mean, 17 mm Hg) at rest and 70/23 mm Hg (mean, 36 mm Hg) during exercise.
People from the mountainous regions of North America represent a genetically mixed community. In comparison with Andean or Tibetan populations, differences in the adaptation to altitude are demonstrable. More remote and longer inhabiting populations perform better than the newly arriving lowlanders. Compared with their Andean or Rocky Mountain counterparts, Tibetans have the following characteristics:
-
Low rates of intrauterine growth retardation
-
Improved neonatal oxygenation, ventilation, and hypoxic ventilatory responses
-
Low pulmonary arterial pressures, hemoglobin values, and rates of chronic mountain sickness
The selection processes reflected in these differences can allow permanent inhabitation at altitudes as high as 5,100 m (16,700 ft). Tibetan teenagers living at high altitude have saturations similar to those of their newly arrived Han counterparts, but they have increased maximal oxygen uptake and increased cardiac output with exercise. Inability to adapt (ie, a deficient ventilatory response to hypoxia and hypercapnia) has been described in a family.
Elite mountaineers who climb Mount Everest without oxygen, as well as some native populations, have an allelic skew, with an excess of the I allele and the II genotype in intron 16 of the human angiotensin-converting enzyme (ACE) gene. Genetic susceptibility to high-altitude pulmonary edema (HAPE) has been attributed to variants of the endothelial nitric oxide synthetase gene (NOS3). Individuals with a genetic risk for increased thrombosis may be at increased risk for HAPE.
Chronic hypoxia, pulmonary venous hypertension, and increased pulmonary blood flow can markedly increase pulmonary pressures in many genetically susceptible individuals; these factors may be additive. At sea level, 25%-30% of adults with critical mitral stenosis and 19% of those with a congenital absence of the pulmonary artery and increased flow to the other lung develop severe pulmonary hypertension. Although pulmonary arterial pressures are elevated, this change does not necessarily cause pulmonary vascular disease.
The 20%-25% of individuals who respond with elevated pulmonary artery pressure at sea level are known as hyperreactors. In such patients, the minimal chronic hypoxia found at even moderate altitude may prime the pulmonary vascular bed and provoke a hyper-reactive response that causes a further increase in hypoxia, increased pulmonary blood flow, or pulmonary venous hypertension. In such cases, the author’s practice is to correct clinically significant cardiac lesions at an early age to allow the pulmonary arterial pressures to regress if continued residence at altitude is contemplated.
Altitude-Related Illnesses
A 2018 systematic review of 13 studies comprising 468 participants found limited evidence to determine the effects of nonpharmacologic and pharmacologic interventions in treating high-altitude illness and indicated high-quality research is needed. [2] The investigators noted low-quality evidence for dexamethasone and acetazolamide suggested these agents may reduce acute mountain sickness score relative to placebo, but their clinical benefits and harms remain unclear.
High-altitude pulmonary edema
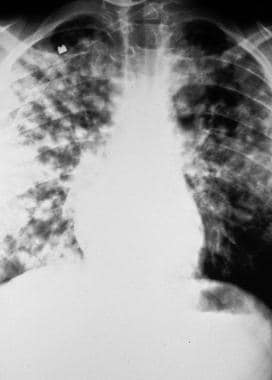
High-altitude pulmonary edema (HAPE) is an unusual form of noncardiogenic pulmonary edema that typically develops in healthy individuals after a rapid ascent to altitudes above 2,500 m (8,200 ft), though the author and others have seen it at lower altitudes (see the images below). Individuals with Down syndrome, obesity, or chronic lung disease may be at an increased risk when they travel to even moderate altitude. HAPE has been reported in both previously healthy high-altitude residents and visitors from lowlands.
It has been suggested that HAPE is often a disease of children living at high altitude. [23] In the series from Leadville, Colorado, 29 of 32 patients with HAPE were younger than 21 years; their mean age was 11.9 years, and they constituted 0.9% of the population aged 1-14 years. The incidence of HAPE above 2,500-5,000 m (8,200-16,400 ft) has been reported to be 0.5%-15.5%, depending on age, sex, history, geographic location, and speed of ascent. The initial or return ascent to altitude is usually rapid and accomplished via either automobile or aircraft.
Sustained exposure to high altitude for 24 hours or longer is usually required for HAPE to develop. Some individuals tolerate high altitude at first but become ill if they attempt to climb to higher altitudes. Fatigue, dyspnea, nausea, and sleeplessness may progress to visible cyanosis, tachypnea, and a productive cough with copious production of pink sputum. Shock and death can result if the symptoms are not recognized and treated.
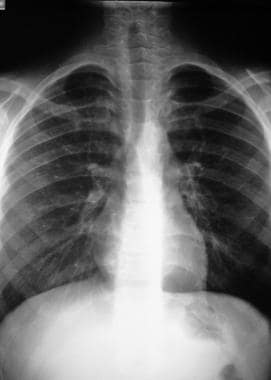
Chest radiography demonstrates patchy infiltrates consistent with pulmonary edema. These rapidly clear, and symptoms improve within 24-48 hours after the start of therapy. The author has treated patients with saturations higher than 60% and severe pulmonary edema on chest radiographs who rapidly improved during a 30-minute ambulance descent from about 2,400-1,800 m (8,000-6,000 ft). During these episodes, electrocardiography (ECG) usually shows evidence of right ventricular (RV) hypertrophy or strain.
After the episode resolves, evidence of RV hypertrophy may or may not persist. The edema fluid is protein-rich, but the primary problem is a hydrostatically induced permeability leak with mild alveolar hemorrhage, which is followed by inflammation. Cardiac catheterization during the acute phase of HAPE reveals pulmonary hypertension with normal wedge or left atrial pressures but increased capillary pressure. This finding supports the concept that HAPE is not related to left ventricular (LV) dysfunction.
A reduced concentration of exhaled nitric oxide, increased plasma endothelin-1 levels, augmented sympathetic activation, and high pulmonary arterial pressures suggest a possible defect in pulmonary nitric oxide synthesis and excessive sympathetically induced hypoxic pulmonary constriction.
HAPE has been described in patients with a congenital absence of the right or left pulmonary artery. In these patients, HAPE develops at moderate altitudes (2,000-3,000 m [approximately 6,500-9,800 ft]). Although this is an uncommon defect, the risk of HAPE in these individuals seems to be extremely high.
Overperfusion edema during uneven vasoconstriction induced by high-altitude hypoxia has been suggested as a possible underlying cause. In addition to focusing on genetic susceptibility, speculation concerning the genesis of HAPE has centered on the role of alveolar hypoxia and overperfusion caused by an uneven hypoxic pulmonary vasoconstriction during periods of exercise at high altitude.
At sea level, the pulmonary vascular pressor response to local pulmonary hypoxia diverts blood to relatively well ventilated areas, improving ventilation-perfusion matching and oxygenation. However, at high altitude, where alveolar hypoxia is global, such a diversion through a universally constricted pulmonary vascular bed is of little benefit.
As noted (see above), pulmonary arterial pressure increases with altitude, and in some patients (ie, hyperreactors), this response is marked. This response can result in severe pulmonary arterial hypertension at rest or with exercise. An increase in pulmonary vasoreactivity has been documented in children and adults in whom HAPE was diagnosed.
In addition to heightened pulmonary vasoreactivity, a reduced hypoxic ventilatory response has been described in patients who have had HAPE. In such individuals, failure to increase the tidal volume and respiratory rate at high altitude further lowers their alveolar oxygen tension (PAO2) and further increases their pulmonary vascular resistance.
High protein concentrations in the lavage fluid suggest a capillary leak. Some assert that the high pressure resulting from the increased pulmonary arteriolar resistance distends the endothelial pores, yielding an exudate of plasma-rich fluid. Elevated plasma levels of atrial natriuretic factor and vasopressin have been reported in patients with HAPE.
The ideal approach to HAPE is prevention. Susceptible individuals who are mountaineers should reconsider this pursuit. Early recognition, rest, and immediate descent may yield rapid improvement, usually in 24-48 hours. Administration of oxygen and inhaled nitric oxide is beneficial but cannot replace rapid descent. Prolonged postural drainage in the head-down position, assisted by steady compression of the upper abdomen, was helpful in a person who had HAPE in a remote setting. [24] Also helpful is a recompression chamber (Gamow bag).
Acetazolamide, a potent carbonic anhydrase inhibitor and mild diuretic, is also a respiratory stimulant. It has been helpful in preventing acute mountain sickness, [20] which may be part of the spectrum of high-altitude pulmonary hypertension. The respiratory stimulant effect of the drug is presumably the reason for its beneficial effect.
Treatment with nifedipine has been reported, and this agent has been used prophylactically with some success. Likewise, favorable effects of prophylactic salmeterol inhalation in susceptible individuals has been reported. Potent diuretics (eg, furosemide) have the theoretical disadvantage of further depleting the already decreased intravascular volume. Morphine may further depress ventilation.
Sildenafil has shown promise in the prevention and treatment of HAPE. Its vasodilatory effects effectively counteract the hypoxia-induced increase in pulmonary arterial pressure. Among study subjects in the French Alps (4,350 m [about 14,300 ft]), sildenafil decreased pulmonary arterial pressure, increased blood oxygenation, and improved exercise performance. [25]
The reader is referred to a recent review of the management of HAPE for an update. [26]
Acute mountain sickness
Each winter, millions of people ski at altitudes of 2,500-3,500 m (8,200-11,500 ft) in Colorado. Each summer, more than 250,000 people visit the summit of Pikes Peak (4,300 m [14,100 ft]). Upon arriving at high altitude, most individuals note a sensation of breathlessness due to the hypoxia-induced hyperventilation and palpitations from the increased heart rate. These are normal physiologic responses.
However, within 6-96 hours of arrival, many individuals have headache, insomnia, anorexia, nausea, vomiting, dizziness, dyspnea, and loss of coordination. These symptoms represent acute mountain sickness, a spectrum that in its severest form can manifest itself as HAPE or high-altitude cerebral edema (HACE). Obese patients and those with chronic lung disease are at particular risk, even at moderate altitudes. For most individuals, the symptoms are annoying but not incapacitating. The duration of symptoms is brief (usually, only a few days).
Data from hikers in Nepal indicated that the overall incidence of acute mountain sickness was 43%-53%. [27] Surveys in Colorado ski areas revealed an incidence of 25% in both adults and children. [28, 29]
The development of acute mountain sickness is directly related to the speed and height of ascent and inversely related to age. The disease is most common in the young. Symptoms observed in preverbal children include increased fussiness, decreased appetite, poor sleep patterns, and decreased playfulness. With serious forms, symptoms include headache, vomiting, ataxia, lassitude, and reduced urination. Rales, peripheral edema (often periorbital), and retinal hemorrhages are sometimes found in affected hikers in Nepal or skiers in Colorado.
Accompanied descent, oxygen, and treatment with acetazolamide are recommended. Acetazolamide 250-500 mg given at bedtime, with or without theophylline 500 mg, is also helpful in relieving the headaches and insomnia in mild cases.
Prophylactic dexamethasone 4 mg given every 6 hours for 6 doses decreased the symptoms of acute mountain sickness in subjects at 2,700 m (8,858 ft) but had no effect at 2,050 m (6,726 ft). [30] In one study, dexamethasone reduced symptoms but did not improve physiologic abnormalities. [31] Another study showed that a combination of low-dose dexamethasone and acetazolamide was superior to acetazolamide alone.
Ginkgo 60 mg given 3 times a day before ascent, aspirin, ibuprofen, and acetaminophen have also been effective in susceptible individuals. Persons who have recovered from any degree of acute mountain sickness can usually reascend at a slow rate.
Oliguria and the retention and redistribution of bodily water into the intravascular and extravascular spaces of the cerebral and pulmonary circulations appear to occur during episodes of acute mountain sickness. Therefore, avoiding dehydration and alcohol consumption are important preventive measures.
Although several hypotheses regarding the cause of HAPE have been offered, the etiology of acute mountain sickness and HACE remains to be elucidated. A low acute hypoxic ventilatory response and hypoxic depression of ventilation have been described in mountain climbers with a history of acute mountain sickness.
High-altitude cerebral edema
Acute mountain sickness that progresses to confusion and neurologic symptoms has been designated as HACE. Early changes include impaired performance and altered behavior. Additional symptoms include headaches, nausea, and vomiting that progress to ataxia, confusion, hallucinations, disorientation, focal neurologic findings, coma, and death. This sickness may develop over a few hours if early symptoms are ignored.
Oxygenation and immediate descent are required. During descent, dexamethasone may be administered. [20] In affected individuals, magnetic resonance imaging (MRI) shows reversible white matter edema, especially in the splenium of the corpus callosum, without abnormalities of the gray matter.
Chronic mountain sickness (Monge disease)
An increase in red blood cell (RBC) production and resulting polycythemia are normal responses to high altitude. However, some high-altitude residents develop excessive polycythemia and become severely symptomatic as a result of polycythemia. This condition has been called chronic mountain sickness, or Monge disease.
Symptoms of Monge disease range from diminished mental and physical capacity to headaches, personality changes, unconsciousness, and coma. Whereas healthy men at 4,540 m (14,900 ft) have hematocrits of approximately 60% and hemoglobin values of 19 g/dL, patients with Monge disease may have hematocrits as high as 84% and hemoglobin concentrations of 28 g/dL. Normal arterial saturation at that altitude (ie, 81%) may fall as low as 60% in adversely affected individuals.
Periods of hypoventilation and arterial desaturation during sleep, as well as small tidal volumes, were described in residents of Leadville, Colorado, with excessive polycythemia. In theory, this desaturation further decreases both alveolar and systemic oxygen tensions and stimulates RBC production.
Blood viscosity increases with the hematocrit, which in turn decreases cerebral blood flow. The worsened hypoxemia due to hypoventilation and the reduced cerebral blood flow combine to cause symptoms. Phlebotomy or erythropheresis improves symptoms and oxygen saturations, and it lowers pulmonary arterial pressure.
Similar increases in erythropoietin values, hematocrits, and blood viscosity have been described in patients with uncorrected cyanotic congenital heart disease.
Perinatal Medicine at Altitude
Pregnancy and childbirth
Travel by pregnant women from low to high altitudes or from high to low altitudes can induce premature labor because of the effect of changing barometric pressures on the amniotic sac. Therefore, this type of travel is discouraged in high-risk women.
A progressive decrease in birth weight with rising altitude has been noted. Changes in the placenta occur in response to lowered maternal arterial oxygen concentrations, and the incidence of preeclampsia is heightened. Gestational age at the time of delivery is not affected; this finding implies intrauterine growth retardation.
Compared with mothers who deliver large babies, mothers of low-birth-weight infants hypoventilate and have decreased oxygen saturations from early to late gestation. Maternal smoking at high altitude is associated with a 2- to 3-fold reduction in the baby’s birth weight in comparison with maternal smoking at sea level.
Pregnant women at high altitude who have an increased ventilatory response to hypoxia augment the normal increase in maternal ventilation associated with pregnancy; thus, they improve their arterial saturations and produce heavier babies than they otherwise would. Of interest, these same women, when they were not pregnant, have the typical blunted hypoxic ventilatory response observed in long-term residents.
Infancy
Infants at sea level have oxygen saturations of 96%-98% within hours of birth. In Denver (1,610 m [5,300 ft]), the mean saturation is 92%-93% at rest, decreasing to 85% during sleeping and feeding. In Leadville, Colorado (3,100 m [10,200 ft]), the highest saturation was 87%-90% during the first 48 hours. It then declined for several weeks and returned to birth levels. During the first 4 months of life at 3,000 m (9,800 ft), mean arterial oxygen saturations are 80%-91%.
Delayed neonatal transition, varying cardiopulmonary pathology, persistent right ventricular (RV) predominance, and subacute high-altitude pulmonary hypertension have also been reported in neonates at high altitude. [15]
Healthy full-term infants born in Cerro de Pasco, Peru (4,340 m [14,200 ft]), had mean saturation values of 43%, 72%, and 88% (maximal) at 1 minute, 15 minutes, and 30 minutes of life, respectively. [32] By comparison, a control group in Lima, Peru (150 m [500 ft]), achieved near-maximal oxygen saturations by 15 minutes of life.
The lowered oxygen levels are associated with an elevated incidence of periodic breathing and apnea. Oxygen administration relieves these symptoms. Many infants are discharged from the author’s nursery with oxygen supplementation during the first few months of life to manage clinically significant apneic episodes.
The data regarding a potentially increased incidence of sudden infant death syndrome at altitude are conflicting. High incidences of hyperbilirubinemia at 3,100 m (10,200 ft) [33] and elevated neutrophil counts in the neonate at various altitudes have been reported. [34] In older children, mean oxygen saturations are 92% at 2,800 m (9,200 ft) and 87% at 4,018 m (13,200 ft).
The fall in the pulmonary arterial pressure from systemic fetal levels to adult levels can be delayed in newborns at high altitude. [35] At high and moderate altitudes, a few infants have a delayed transition from fetal hemodynamics. Many of these infants are at risk for pulmonary vascular hyperreactivity (eg, Down syndrome) or were subjected to perinatal stress.
On examination, findings in these infants are generally unremarkable, aside from moderate cyanosis (peripheral saturation, 80%-90%), slight tachypnea, and a single loud second heart sound. Chest radiographs are normal. Normal resting arterial oxygen saturations are easily achieved after administration of oxygen at 0.5-1 L/min.
Oxygen administration is usually required for 4-6 weeks. In that time, findings on clinical examination usually suggest a decrease in the pulmonary arterial pressure. However, RV dominance on the electrocardiogram (ECG) usually resolves more slowly than the clinical abnormalities do. Noninvasive studies usually suffice to exclude other causes of cyanosis in the newborn.
The response of these patients to exercise at high altitude when they are older has not been established. The infants’ conditions may reflect a benign spectrum of the more serious and persistent fetal circulation syndrome or, as is more likely, may represent an exaggeration of the normal delayed fall in pulmonary vascular resistance observed at high altitude.
In a few infants, the pulmonary hypertension does not resolve or else redevelops after subsequent exposure to altitude; these infants may experience failure to thrive and cor pulmonale. This condition has been referred to as symptomatic HAPE (SHAPE) or subacute infantile mountain sickness (SIMS). Infants with SHAPE or SIMS must be moved to a low altitude.
Human and animal data suggest that perinatal exposure to hypoxia may manifest as lifelong exaggerated hypoxic pulmonary vasoconstriction. Therefore, the author follows patients with such findings during the first years of their lives and cautions their parents to avoid taking their infants to the summit of Pikes Peak (4,303 m [14,100 ft]).
Oxygen saturations in healthy infants residing in or traveling to Summit County, Colorado, a popular ski resort with an altitude of 2,800 m (9,200 ft), are 88%-97% (mean, 91.7%). In Bolivia, near La Paz, at an altitude of 4,018 m (13,200 ft), the mean saturation is 87.3%.
Congenital cardiac anomalies
Patent ductus arteriosus, atrial septal defect, and anomalies of the branchial arch are all more common at altitude. In school-aged children living at high altitude, the incidence of patent ductus arteriosus is 18-30 times that noted at sea level.
In the author’s experience, the incidence of patent ductus arteriosus at moderate altitude is only minimally elevated. However, closure of the ductus, as assessed with Doppler echocardiography, is often delayed for 7-10 days among infants in the neonatal intensive care unit. Accordingly, the author does not recommend immediate medical or surgical intervention to close the ductus in infants, except in those with signs of clinically significant volume overload.
Altitude and Congenital Heart Disease
Increased pulmonary blood flow
The reactivity of the pulmonary vascular bed is known to vary widely from one person to another. In healthy people, pulmonary arterial pressure does not substantially increase until alveolar oxygen tension (PAO2) falls below 65 mm Hg. However, in people with reactive pulmonary vasculature and a chronic stimulus to maintain reactivity (eg, increased pulmonary blood flow or pulmonary venous hypertension), even the minimal hypoxia due to a moderate increase in elevation may be enough to stimulate a substantial increase in pulmonary vascular resistance.
In Denver, 34 infants with a ventricular septal defect and pulmonary hypertension were compared with 54 infants at sea level at Texas Children’s Hospital. [16, 17] Despite similar pulmonary arterial pressures, the infants at sea level had half the vascular resistance that the infants from Denver had.
The hypoxic banding of the pulmonary artery is responsible for the relative infrequency with which infants with refractory congestive failure from left-to-right shunts at moderate altitude are encountered. However, this situation is a double-edged sword. A thriving infant may have a clinically significant defect with severe pulmonary arterial hypertension.
Consequently, examination of the right ventricular precordial impulse, assessment of the second heart sound, electrocardiography (ECG), and echocardiography are extremely important. The author has heard a loud murmur in a patient at a relatively low altitude that could not be heard during an examination at a higher elevation.
Because of anecdotal reports of irreversible pulmonary vascular obstructive disease in children younger than 2 years living at moderate altitude, early repair is recommended for children who have clinically significant pulmonary arterial hypertension in infancy.
Administration of oxygen or nitric oxide in the catheterization laboratory may help document a decrease in pulmonary vascular resistance. However, the decrease, as indicated by an increase in the intensity of a murmur and by clinical signs of an increasing left-to-right shunt, sometimes does not occur until after several days of oxygenation. Therefore, for appropriate individuals, oxygen is administered at home 1 week before the planned catheterization procedure to achieve maximal reduction of pulmonary vascular resistance.
In addition, reversible causes of chronic hypoventilation (eg, tonsil or adenoid hypertrophy) are corrected before any decision is made regarding a patient’s suitability for surgery. Several patients for whom surgery was deemed inappropriate at moderate altitude, even after oxygenation, had decreased pulmonary vascular resistance when they were transferred to sea level, and their condition was successfully repaired.
For pulmonary vasodilation, administering oxygen by conventional means is evidently not as effective as raising the barometric pressure. After returning to moderate altitude, patients often have persistent hyperreactivity to hypoxia and exercise. Recommendations regarding high-altitude exercise (eg, skiing) for this group of patients are lacking.
Patent foramen ovale
Patent foramen ovale may predispose an individual to high-altitude pulmonary edema (HAPE). The size of the patent foramen ovale seems to be clinically significant. [36] It is plausible that right-to-left shunting occurs across a large patent foramen ovale in the setting of increased pulmonary arterial pressure and that this shunt contributes to relatively severe hypoxemia and worsening HAPE.
Cyanotic lesions
Data regarding individuals with right-to-left shunts at altitude are limited. The incidence of symptomatic polycythemia and the frequency of phlebotomies do not appear to be higher at moderate altitude than at sea level. Exercise is undoubtedly more limited at high altitude than it is at sea level, but objective data are lacking.
Recommendations regarding travel to high altitude for patients with cyanosis are based on whether the cyanosis is associated with increased or decreased pulmonary blood flow, on the systemic saturation, and on the patient’s hematocrit at moderate altitude. It is important to consider whether the patient will be exercising or inactive. Overnight stays increase the patient’s risk because of the potential increase in desaturation associated with hypoventilation during sleep. Supplemental oxygen can be provided for weekend trips.
Pulmonary vascular obstructive disease
Because mild chronic hypoxia stimulates pulmonary vascular hyperreactivity, the progression of pulmonary vascular obstructive disease is thought to be accelerated at moderate or high altitude. However, data are lacking. If social circumstances are favorable, patients with this condition are encouraged to move to lower altitudes. They are less symptomatic at sea level than at altitude, but whether the disease process is altered is unknown.
Case reports describe children from Leadville, Colorado, who had primary pulmonary hypertension. Their condition improved after they moved to lower altitudes. The disease did not have the usual progression after the change in location. However, 1 patient died after a similar move.
Fontan procedure candidates
Patients undergoing the Fontan procedure are uniquely sensitive to the effects of an increased pulmonary vascular resistance. Ideal candidates for the Fontan procedure have the same survival rates at moderate altitude that they do at sea level. However, their exercise tolerance is lowered at moderate altitude. In the author’s experience, several patients who did not meet the hemodynamic criteria for surgery at moderate altitude were treated with recatheterization at sea level. The operations were successful.
The postoperative course at sea level has been typical for children undergoing this operation. After a return to moderate altitude, however, many of these patients’ conditions deteriorate acutely. Whether patients whose cardiac output is sensitive to pulmonary vascular resistance should live or even visit regions at moderate altitude is questionable. As always, patients with the best operative results have the most flexibility in this regard.
Chronic Lung Disease and Hemoglobinopathies
Chronic lung disease
Chronic pulmonary disease can aggravate the hypoxia of increased altitude. Survival data for patients with chronic diseases (eg, cystic fibrosis) who live at altitude are lacking. The incidence of bronchopulmonary dysplasia among infants does not appear to be significantly increased at moderate altitudes, though long-term care of these patients usually involves prolonged oxygen therapy to maintain adequate saturations.
Chronic hypoxia associated with cystic fibrosis may contribute to the development of retinopathy at a relatively low altitude, in comparison with that seen in high-altitude hikers. Rimsza et al described hemorrhagic retinopathy and symptoms similar to those of acute mountain sickness at 3,049 m (10,000 ft). [37] They speculated that other individuals with chronic hypoxia may be at a similar risk.
Altitude and hemoglobinopathy
Altitude-induced hypoxic stimulation of sickle cell crisis has been studied in patients in Colorado. [38] Travel above 2,000 m (6,500 ft) poses an estimated 20%-30% risk of crisis in persons with hemoglobin SS, hemoglobin S/C, or hemoglobin S/T.
Residing in Denver is not thought to increase the incidence of problems. In the literature, cardiopulmonary function in men with sickle cell trait who resided in Denver was reported to be equal to that in control subjects. [39]
Splenic sequestration syndrome in patients with sickle–hemoglobin C disease has been described both during air travel in unpressurized cabins and at moderate altitude. [40] In patients with the sickle cell trait, altitude-induced splenic syndrome (acute left upper quadrant pain and tender splenic enlargement) appeared to be less common in African Americans than in people of other races. This finding was in contrast to the generally benign nature of sickle cell trait in African Americans at moderate altitude.
Air Travel
Air travel is a common mode of transportation for patients with congenital heart disease or chronic pulmonary disease who are traveling to and from major medical centers. Factors unique to this mode of transportation include the cabin pressure at altitude, the acceleration-deceleration forces, and the increases in gas volumes associated with reduced cabin pressure.
All aircraft with pressurized cabins can maintain a certain pressure differential with respect to the outside atmosphere, but they do not maintain a sea-level pressure of 760 mm Hg. Most commercial aircraft are designed to keep their cabin pressure at no lower than 565 mm Hg (equivalent to an altitude of 2,400 m [8,000 ft]) at maximum operating altitude. However, on flights above 6,860 m (22,500 ft) altitude, the cabin pressure may be equivalent to that encountered at altitudes as high as 2,700 m (8,915 ft).
Oxygen saturation decreases by approximately 4% at 2,100-2,400 m (7,000-8,000 ft). According to an article in the New England Journal of Medicine, this level of desaturation does not produce symptoms of high-altitude pulmonary edema (HAPE) or acute mountain sickness in healthy unacclimatized adults. [41]
At cabin pressures equivalent to an altitude as high as 2,700 m (8,915 ft), alveolar oxygen tension (PAO2) is 59 mm Hg and arterial oxygen tension (PaO2) 55 mm Hg in a healthy individual. In a patient with a resting PaO2 of 50 mm Hg at sea level, PaO2 may decrease to 30 mm Hg during air travel. In patients with reactive pulmonary hypertension, pulmonary arterial pressures may substantially increase during a flight, and hypoxemia may increase.
Anecdotal reports describe deterioration in patients with unrepaired total anomalous venous connection during air travel. [42] Patients with Eisenmenger syndrome may have exaggerated decreases in saturations with minimal exertion while in flight.
All patients with cystic fibrosis, chronic emphysema, cyanotic congenital heart disease, severe chronic asthma, coronary insufficiency, fibrotic pulmonary changes, or a PaO2 lower than 50 mm Hg should receive supplemental oxygen during flights above 6,900 m (22,500 ft). However, if a patient resides at 1,900 m (6,200 ft) and tolerates that altitude well, little change occurs during flight, as the cabin pressure varies little from the local ambient barometric pressure.
Adults with stable cyanotic congenital heart disease or irreversible pulmonary hypertension have traveled on commercial airlines without consequence. This finding is probably because most air travel is sedentary and relatively brief.
Commercial airlines do not carry therapeutic oxygen systems sufficient to sustain a patient on anything more than an emergency basis. Many, but not all, airlines allow patients to carry on oxygen supplies if they arrange for this well in advance. The Federal Aviation Administration (FAA) has strict rules regarding the type of systems allowed on an aircraft. If connecting flights at moderate-altitude airports are to be taken, planning for additional oxygen supplies and a wheelchair to reduce the patient’s exertion may be indicated.
Another concern is the risk of stroke or thromboembolism during long flights. Patients with polycythemia may be at an increased risk from the effects of dehydration associated with air travel. Therefore, adequate anticoagulation and hydration are considerations.
Patients on stretchers can be accommodated in the first-class section by having the airline staff remove seats. In commercial aircraft, acceleration and climb angle have little effect on healthy sitting passengers. A prone or supine patient whose head is toward the front of the aircraft can experience considerable venous pooling and decreased cardiac output during takeoff. This is a theoretical concern for some patients with cardiac conditions. Therefore, the patient’s head is positioned toward the rear of the aircraft (except if he or she has cerebral edema).
The reduction in cabin pressure associated with air flight allows gas volumes to expand (Boyle law). For this reason, a pneumothorax or congenital cyst of the lung may enlarge and complicate air travel; therefore, commercial air travel is not recommended for patients with such conditions. Flying should be deferred for 2 weeks after gastrointestinal or genitourinary surgery.
-
High-altitude pulmonary edema (HAPE). Initial presentation at 8,200 ft.
-
High-altitude pulmonary edema (HAPE). Improvement within 24 hours after descent to 6,200 ft.