Background
Pacemaker therapy in children involves unique issues regarding patient size, growth, development, and the possible presence of congenital heart disease; accordingly, this topic reviews aspects of pediatric pacemaker implantation and follow-up, with particular attention to the difficulties encountered with smaller children and patients with coexistent congenital heart defects. [1, 2, 3, 4, 5]
Transvenous pacemaker implantation in young patients was previously limited by generator size and lead diameter in comparison to vascular dimensions and capacitance. Historically, epicardial pacing was more common in children. As technology has improved, generators and leads have become smaller and more advanced, allowing transvenous pacing systems in children; pacemaker therapy is now possible in neonates.
Pediatric pacemaker implantation is performed primarily to treat abnormalities of sinoatrial (SA) node (ie, sinus node) or atrioventricular (AV) node function that lead to an insufficient heart rate. In addition, pacemakers are used (although less commonly) for other disorders, such as congenital long QT syndrome and cardiomyopathy. A specialized type of pacemaker therapy known as cardiac resynchronization therapy (CRT) is used adjunctively in pediatric patients with heart failure. [6, 7] In addition, although relatively rare and decreasing in incidence, acute permanent pacemaker implantation after heart transplanation in children can occur and is associated with biatrial anastomosis, antiarrhythmic use, and older donor age; there is also an increased risk for posttransplant infection and dialysis but survival does not appear to be adversely affected by permanent pacemaker implantation. [8]
Chronotropic incompetence is the term used to describe the inability of the SA node to increase heart rate adequately as needed for the degree of activity. Sinus node dysfunction is a related term that describes an inappropriately low heart rate (either sinus or nonsinus) due to abnormal activity of the normal SA node.
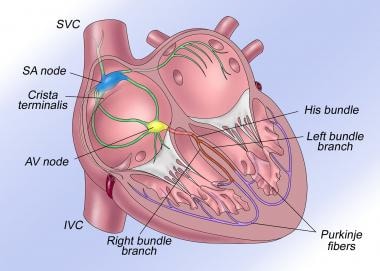 The normal cardiac conduction system is illustrated. AV = atrioventricular, IVC = inferior vena cava, SA = sinoatrial, SVC = superior vena cava.
The normal cardiac conduction system is illustrated. AV = atrioventricular, IVC = inferior vena cava, SA = sinoatrial, SVC = superior vena cava.
AV blocks occur in three degrees of severity, as follows:
-
First degree: The impulse is conducted through the AV node, albeit delayed.
-
Second degree: Not all impulses are conducted through the AV node.
-
Third degree: AV block is complete, and none of the impulses are conducted across the AV node.
Causes of SA or AV node dysfunction can be divided into two distinct categories: congenital and acquired. Congenital causes include congenital AV block and congenital SA node dysfunction, which is notably less common. Congenital heart block is often due to autoantibody production from maternal systemic lupus erythematosus (SLE). [9] Also, congenital heart block may be associated with congenital heart disease, such as L-transposition of the great arteries.
Acquired forms of heart block or SA node dysfunction are caused by infection or injury, whereas other forms are idiopathic. Infections include viral myocarditis and Lyme disease. Surgical repair of congenital heart disease is the predominant cause of nodal or conduction tissue injury.
Permanent pacing in children is a successful procedure that results in physiologic heart rates. Pacing also allows the pediatric patient to return to normal activity and lifestyle. The prognosis is excellent, because modern generation pacing systems allow physiologic atrial-synchronous, rate-responsive pacing in the vast majority of patients, even small infants and children with congenital heart disease.
Indications and Contraindications
Indications
Although indications for pacing in children differ from those in adults, both include abnormalities in sinoatrial (SA) node and atrioventricular (AV) node function. The American Heart Association (AHA) and the American College of Cardiology (ACC) publishes guidelines and updates for implanting pacing systems in children. [10, 11, 12, 13] The AHA’s scientific statements on pacing in children and adolescents divide the recommendations for pacemaker implantation in children into several classes reflecting the strength (or the absence) of the indication. [13] These indications continue to evolve as the definition of the natural history of the disease improves and pacemaker technology and diagnostic methods advance.
Class I signifies that pacing is indicated and includes the following [13] :
-
Advanced second- or third-degree AV block with symptomatic bradycardia, ventricular dysfunction, or low cardiac output
-
SA node dysfunction with symptoms during age-inappropriate bradycardia (The definition varies with the patient’s age and expected heart rate.)
-
Postoperative advanced second- or third-degree AV block that is not expected to resolve or that persists for at least 7 days
-
Congenital third-degree AV block with a wide QRS escape rhythm, complex ventricular ectopy, or ventricular dysfunction
-
Congenital third-degree AV block in an infant with a heart rate lower than 55 beats/min or with congenital heart disease and a heart rate below 70 beats/min
Class IIa signifies that there is general agreement that pacing is indicated and includes the following [13] :
-
Prevention of recurrent episodes of intra-atrial reentrant tachycardia in patients with congenital heart disease and sinus bradycardia (Sinus node dysfunction may intrinsic or due to antiarrhythmic therapy.)
-
Congenital third-degree AV block beyond the first year of life, with an average heart rate lower than 50 beats/min, abrupt pauses in ventricular rate that are 2-3 times the basic cycle length, or symptoms due to chronotropic incompetence
-
Sinus bradycardia in a child with complex heart disease, with a resting heart rate lower than 40 beats/min or pauses in ventricular rate exceeding 3 seconds
-
Patients with congenital heart disease and impaired hemodynamics due to sinus bradycardia or loss of AV synchrony
-
Unexplained syncope in a patient with a prior congenital heart surgery complicated by transient complete heart block with residual fascicular block
Class IIb signifies that although there is no consensus, implantation may reasonably be considered for the following [13] :
-
Transient postoperative third-degree AV block that reverts to sinus rhythm with residual bifascicular block
-
Congenital third-degree AV block in an asymptomatic infant, child, or young adult with an acceptable rate and function and narrow QRS
-
Asymptomatic sinus bradycardia following biventricular repair of congenital heart disease, with a heart rate lower than 40 beats/min or pauses in ventricular rate exceeding 3 seconds
Contraindications
Class III signifies that pacing is not indicated and includes the following [13] :
-
Transient postoperative AV block with return of normal AV conduction in an otherwise asymptomatic patient
-
Asymptomatic postoperative bifascicular block with or without first-degree AV block
-
Asymptomatic type I second-degree AV block
-
Asymptomatic sinus bradycardia with the longest relative risk interval shorter than 3 seconds and a minimum heart rate higher than 40 beats/min
In addition, expected survival of less than 6 months is a relative contraindication to permanent pacing therapy for patients who are terminally ill. Patients who fully regain normal conduction after transient postoperative heart block typically do not need to receive a permanent pacemaker.
Technical Considerations
Best practices
Congenital (or acquired) structural heart disease presents additional issues for pacemaker implantation in children. These patients may have a more critical reliance on adequate hemodynamic status. Optimal hemodynamic performance is achieved with atrial synchronous pacing.
Maintaining atrioventricular (AV) synchrony in this population may be more important than in children who have structurally normal hearts. For example, in a newborn with congenital complete heart block but a structurally normal heart, an epicardial ventricular pacing system initially suffices to meet hemodynamic needs. In contrast, a newborn with significant structural heart disease, heart block, and congestive heart failure may benefit more from a dual-chamber system to obtain AV synchrony and meet hemodynamic needs.
In general, the transvenous route is a reasonable approach for children weighing at least 10-15 kg, although successful transvenous pacing is reported in neonates without complications. Physical considerations that may preclude transvenous pacing include intracardiac shunting, low-flow states, and anatomic barriers (eg, mechanical tricuspid valves).
Transvenous lead placement in congenital heart patients often requires nonstandard positioning because of variations in venous and intracardiac anatomy. The atrial appendage is sometimes amputated with cannulation for cardiac bypass, and atrial anatomy is often different. The use of active-fixation leads allows easier sampling of nonstandard pacing sites and easier removal.
In specific operations for congenital heart disease, such as the Fontan procedure, the right medial wall is often viable; in postoperative Mustard and Senning procedures, superior aspects of the systemic venous atrium are optimal. High-output pacing is imperative testing for diaphragmatic or phrenic nerve stimulation, particularly in lateral pacing sites. The active pacing lead tip may be used for mapping of optimal tissue implant sites.
Although anticoagulation is not universally recommended, it may help patients who have leads implanted in low-flow chambers. It is also important to consider the possibility of future rhythm complications in children who have undergone palliative repairs. For example, a child who develops heart block after a Fontan procedure needs ventricular pacing. These children are also at risk for sinoatrial (SA) node dysfunction; thus, placing an epicardial atrial pacing lead concurrent with placing a ventricular lead may prove beneficial in the future.
Certain populations are at greater risk for the development of atrial tachycardia; therefore, pacemaker type is important. A patient with SA node dysfunction who is at risk for atrial tachycardia should probably have a generator placed that also allows antitachycardia pacing, either manual or automatic. There are automatic atrial antitachycardia pacemakers that have been shown to be effective in pediatric congenital heart disease patients.
For those patients with heart failure, cardiac resynchronization therapy (CRT) may be used in conjunction with medical therapy to improve cardiac function and quality of life. Although indications for CRT in pediatrics remain controversial, important anatomic considerations are noted. CRT requires two strategically placed leads in the ventricles (typically one in the right ventricle and one in the left ventricle) that pace two ventricular areas to synchronize ventricular contraction. A transvenous approach requires a patent coronary venous system that is accessed via the coronary sinus. Typically, the left ventricular lead is placed through the coronary sinus and then carefully maneuvered into one of the tributary cardiac veins of the left ventricle. Patients with congenital heart disease often have distorted coronary venous anatomy; in these patients, CRT may require an epicardial approach. A hybrid approach may be considered in select patients, such as dextro-transposition of the great arteries (d-TGA) following Mustard or Senning repair where transvenous leads are placed in the systemic venous atrium and pulmonary ventricle with an epicardial lead placed on the systemic ventricle.
Procedural planning
Single-chamber versus dual-chamber pacing
Whether dual-chamber synchronous pacing (see the image below) is superior to single-chamber ventricular pacing remains a subject of debate. [14, 15, 16, 17] Atrial-based pacing, which includes AAI(R), DDD, and VDD(R) pacing modes, has been shown to be superior to ventricular-based (VVIR) pacing in studies of adults, although controversy regarding DDD versus VVI pacing continues. Several large-scale prospective studies are attempting to illuminate these issues.
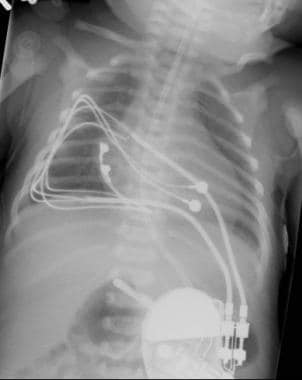 An epicardial dual-chamber implantable cardioverter defibrillator is shown in a neonate with congenital complete atrioventricular block. Two bipolar suture-on leads (one on the atrium and one on the ventricle) are attached to the DDDR pacemaker in the abdomen.
An epicardial dual-chamber implantable cardioverter defibrillator is shown in a neonate with congenital complete atrioventricular block. Two bipolar suture-on leads (one on the atrium and one on the ventricle) are attached to the DDDR pacemaker in the abdomen.
In adult studies, dual-chamber pacing may have lower morbidity and mortality compared with ventricular pacing in patients with congestive heart failure, valvular heart diseases, and hypertensive heart disease. Studies indicate higher incidences of death, stroke, atrial fibrillation, pacemaker syndrome, and heart failure during VVI pacing than during DDD pacing.
An apparently convincing argument advocates atrial-based pacing (either single-chamber AAI[R]) or dual-chamber modes) over ventricular-based pacing modalities, although whether DDD is better than AAI(R) for patients with intact AV conduction is less clear.
Experimental data that address pacing in children are more limited, and some conclusions are extrapolated from adult patient series. Common pacing indications in children are similar to those in adults (eg, SA or AV node dysfunction), although concomitant cardiac, medical, psychological, and size-related issues often differ markedly. Rate-responsive ventricular pacing often has been used in children with complete AV block. Rate-responsive ventricular pacing also responds adequately to the physiologic requirements of healthy active children.
Many pediatric patients, however, have underlying structural heart disease, tachyarrhythmia, and hemodynamic derangements that compromise ventricular performance. In addition, pediatric patients frequently have structural anatomic barriers to implantation and limited access to cardiac chambers.
Many patients who have previously undergone atrial surgical procedures (eg, Fontan, Mustard, or Senning procedures) have both bradyarrhythmia and tachyarrhythmia. Some studies have demonstrated higher atrial-pacing failure rates in these patients, presumably as a consequence of higher atrial pressures, scarring, and ischemia.
A large retrospective series revealed no significant differences between many patients who had undergone Fontan or atrial switch procedures. [18] Investigators compared these patients with others who underwent Fontan surgery and received no permanent pacemaker, an atrial single-chamber pacemaker, a ventricular-based pacing system, or a dual-chamber pacing system. No clear choice of pacing modality emerged for pediatric single-ventricle patients. [18] The use of multisite pacing for cardiac resynchronization therapy in single-ventricle patients has been used in multiple centers with improved hemodynamics compared to the traditional single-site pacing. [19, 20]
Epicardial versus endocardial pacing sites
The main difference in lead implantation is the route of placement. Although the vast majority of adult pacemaker patients have transvenous lead placement, children have an almost even distribution of transvenous and epicardial lead implantation.
For some time, epimyocardial pacing was the most common pediatric pacing application. [21] At present, epicardial pacing is primarily used when transvenous pacing is contraindicated or for patients undergoing concomitant heart surgery. Contraindications to transvenous pacing include prosthetic tricuspid valves, right-to-left intracardiac shunts, congenital heart disease, surgery precluding transvenous access to cardiac chambers, recurrent transvenous lead dislodgment, and, probably, minimum patient size.
Advantages of epicardial implantation include the absence of a need to provide vascular continuity with the cardiac chambers and the avoidance of concerns about venous thrombosis. Disadvantages include more frequent reports of sensing and capture failure, higher rates of insulation and conductor fractures, and the need for an open chest approach (eg, via a thoracotomy, a sternotomy, or a subxiphoid or subcostal incision). However, less-invasive surgical options are available in the pediatric population, including utilization of a mini-thoracotomy or subxiphoid approach and pericardial window for pericardial lead placement.
Transvenous pacing is feasible in infants and small children. Smaller pacemaker generators and thinner lead diameters simplify the placement of permanent transvenous pacing systems. However, the easier placement of transvenous leads in small patients does not imply that this approach is necessarily superior to others.
Current practice suggests that transvenous pacing leads routinely can be placed in children who weigh more than 10 kg, although there are reports of centers performing transvenous implants in children weighing less than 10 kg with good outcomes. [22] There is even a case report of a successful pacemaker implant for complete heart block in a low birth weight infant (803 g). [23] As pacing technology continues to reduce lead body diameter, it is likely that children with even smaller body weights can be considered for transvenous placement. However, because of continued growth and vigorous activity, pediatric patients have distinctly higher lead fracture and failure rates than adults do.
Actual survival comparisons have been performed for transvenous pacing leads in children. These comparisons show progressive lead failure over time from fracture, insulation discontinuities, adapter or header failures, or pacing exit block.
Advantages of the transvenous route include avoidance of thoracotomy, lower pacing thresholds (and thus longer battery life), and lower incidences of exit block and lead fractures. Disadvantages include a slightly higher dislodgment rate (eg, with passive fixation devices), potential venous occlusion, [24] possible embolic events (eg, from an intracardiac shunt), a slight risk of endocarditis, and subclavian crush syndrome.
Minimally invasive pericardial lead placement, both with pacemaker and defibrillator functionality, have been attempted in an animal model with acute and chronic success, with the potential for future use in the human pediatric population. [25, 26]
Twiddler syndrome, in which permanent malfunction of a pacemaker from manipulation of the pulse generator, can also occur in children with potential lead dislodgment, generator migration, or pacing failure due to twisting of the leads or generator in the pocket. Significant vascular access challenges can also relate to congenital heart diseases and surgical corrections.
-
This electrocardiogram reveals a sinus atrial mechanism with complete atrioventricular block and a ventricular paced rhythm.
-
This electrocardiogram illustrates third-degree atrioventricular block in a 2-year-old child.
-
This electrocardiogram illustrates an atrial-synchronous, ventricular paced rhythm.
-
The normal cardiac conduction system is illustrated. AV = atrioventricular, IVC = inferior vena cava, SA = sinoatrial, SVC = superior vena cava.
-
This radiograph depicts a transvenous ventricular pacemaker in 2-year-old child. Note the abundant slack in the lead to allow for growth.
-
An epicardial dual-chamber implantable cardioverter defibrillator is shown in a neonate with congenital complete atrioventricular block. Two bipolar suture-on leads (one on the atrium and one on the ventricle) are attached to the DDDR pacemaker in the abdomen.






