Background
The American Academy of Pediatrics (AAP) and the World Health Organization (WHO) both recommend exclusive breastfeeding until an infant is at least 6 months of age. [1, 2] Breast milk is composed of many essential substances for optimal growth and development, including carbohydrates, fats, and proteins. The bioactive functions of the proteins in breast milk are especially important during critical periods of brain, gut, and immune development. As a result, breastfed babies have lower rates of otitis media as well as respiratory and intestinal infections. In addition to these short-term benefits, there are many long-term advantages to breastfeeding, including improved cognitive development and a reduced risk of cardiovascular disease, obesity, and type 2 diabetes. Studies have shown that mothers also benefit from breastfeeding, with favorable maternal metabolic changes including lower rates of hypertension, hyperlipidemia, and cardiovascular disease. Moreover, some studies show that breastfeeding can reduce the risk of breast and ovarian cancer. [3, 4]
Despite these overwhelming benefits for both mother and baby, the rates of breastfeeding are still not optimal. Clinicians play a crucial role in a mother's decision to breastfeed as well as her success in lactation. Informing women of the evidence that breast milk contributes to both a baby's short- and long-term well-being as well as its potential health benefits for the mother, clinicians can help them understand its importance. To effectively counsel and educate mothers, it is essential that clinicians be familiar with how the mammary gland produces human milk and how its properties nourish and protect the breastfeeding infant.
This article reviews the development of the mammary gland (mammogenesis), the process through which the mammary gland develops the capacity to secrete milk (lactogenesis), the process of milk production (lactation), and the specific properties of human milk that make it unique and appropriate for human infants. In a related article titled Counseling the Breastfeeding Mother, the mechanics of breastfeeding and evaluation of the breastfeeding mother-infant dyad are discussed. Such articles are intended to be overviews. For a more in-depth treatise, please refer to textbooks by Lawrence and Lawrence (2005) [5] and the AAP's Breastfeeding Handbook for Physicians (2006). [6] Guidelines for breastfeeding and the use of human milk have been established by the AAP. [1]
For patient education resources, see the Pregnancy Center and Breastfeeding.
Physiology
Mammogenesis
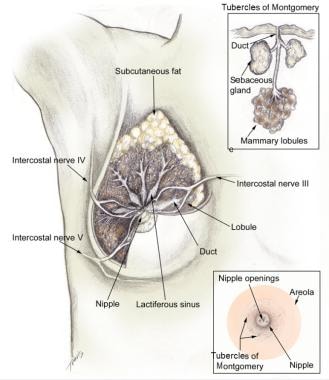
Knowing how the breast develops is important to understanding the physiologic changes that happen in order for lactation to occur. Breast tissue begins to develop in utero, undergoing the first of many developmental changes necessary for proper breastfeeding to occur. At 18-19 weeks' gestation, a bulb-shaped mammary bud can be discerned in the fetus. Inside the bud, a rudimentary mammary ductal system is formed, which is present at birth. After birth, growth of the gland parallels that of the child until puberty. The normal anatomy of the mammary gland following pubertal development is shown in the images below. [7]
The normal breast consists of approximately 15-20 lobes of glandular tissue. These lobes are further divided into lobules that produce milk during and after pregnancy. Each lobe contains 20-40 lobules. The milk produced in the lobules within each lobe empty into a lactiferous duct. The lactiferous ducts then merge into 5-10 main lactiferous ducts, which then open at the nipple, allowing the infant to receive the milk.
Lobule types 1-4
There are four types of lobules. Type 1 lobules are formed in utero and are present until puberty. Once puberty has started, the changes in hormone levels of estrogen and progesterone during each menstrual cycle stimulate type 1 lobules to produce new alveolar buds and evolve to the more mature type 2 lobules. Once puberty is complete, no further changes occur to the female breast until pregnancy.
During pregnancy, circulating hormones lead to a remodeling of the breast, in which the lobules increase progressively in number and size. By the end of pregnancy, the breast is composed almost entirely of type 3 lobules. Once lactation has commenced, the lobules produce and secrete milk and are considered type 4 lobules. When lactation has ceased, the type 4 lobules regress back to type 3 as a result of the cessation of lactogenic hormone stimulation as well as local autocrine signals that result in apoptosis and tissue remodeling.
Lactogenesis
In lactogenesis, the mammary gland develops the capacity to secrete milk. Lactogenesis includes all processes necessary to transform the mammary gland from its undifferentiated state in early pregnancy to its fully differentiated state sometime after pregnancy. This fully differentiated state allows lactation to occur. The two stages of lactogenesis are discussed below.
Stage 1 occurs by mid pregnancy, when the mammary gland becomes competent to secrete milk. Lactose, total protein, and immunoglobulin concentrations increase within the secreted glandular fluid, whereas sodium and chloride concentrations decrease. The gland is now sufficiently differentiated to secrete milk, as evidenced by the fact that women often describe drops of colostrum on their nipples in the second or third trimester. However, high circulating levels of progesterone and estrogen hold the secretion of milk in check.
Stage 2 of lactogenesis occurs around the time of delivery. It is defined as the onset of copious milk secretion due to the rapid drop in progesterone secondary to the removal of the placenta as well as increased levels of prolactin, cortisol, and insulin. Work by Haslam and Shyamala reveals that progesterone receptors are lost in lactating mammary tissues, thus decreasing the inhibitory effect of circulating progesterone. [8, 9] Citrate levels also increase during lactogenesis; this increase is considered a reliable marker for the second stage of lactogenesis.
The stages of lactation can be summarized as follows (adapted from Riordan and Auerbach, 1998). [10]
Mammogenesis
Mammary (breast) growth occurs. The size and weight of the breast increase.
Lactogenesis
-
Stage 1 (mid pregnancy): Alveolar cells are differentiated from secretory cells.
-
Stage 2 (day 2 or 3 to day 8 after birth): The tight junction in the alveolar cell closes. Copious milk secretion begins. Breasts are full. Endocrine control switches to autocrine (supply-demand) control.
Galactopoiesis (later than 9 days after birth to beginning of involution)
Established secretion is maintained. Autocrine system control continues.
Involution (average 40 days after last breastfeeding)
Milk secretion decreases from the buildup of inhibiting peptides.
Lactation
During the second stage of lactogenesis, the breast becomes capable of milk production. For the ongoing synthesis and secretion of human milk, the mammary gland must receive hormonal signals. These signals, which are in direct response to stimulation of the nipple and areola (mammae), are then relayed to the central nervous system. This cyclical process of milk synthesis and secretion is termed lactation. Lactation occurs with the help of two hormones, prolactin and oxytocin. Although prolactin and oxytocin act independently on different cellular receptors, their combined actions are essential for successful lactation.
Prolactin
-
Prolactin is a polypeptide hormone synthesized by lactotrophic cells in the anterior pituitary. The binding of prolactin to receptors on the epithelial cells in the mammary gland stimulates milk production. These receptors are downregulated during periods of elevated progesterone levels such as during pregnancy. Once delivery has occurred and the placenta is removed, the progesterone levels drop and the prolactin receptors are upregulated enabling lactogenesis to take place.
-
Research during the past several decades has led to a deeper understanding of prolactin's role in the body. Prolactin-related knockout animal models support prolactin's pivotal role in lactation and reproduction, which suggests that most of prolactin's target tissues are modulated rather than dependent on prolactin. [11]
Oxytocin
-
The other important hormone involved in the milk ejection or letdown reflex is oxytocin, which stimulates the myoepithelial cells to contract. When the neonate is placed at the breast and begins suckling, oxytocin is released. The suckling infant stimulates the touch receptors that are densely located around the nipple and areola, which then create impulses that activate the dorsal root ganglia via the intercostals nerves. [10, 12, 13] These impulses ascend the spinal cord, creating an afferent neuronal pathway to the paraventricular nuclei of the hypothalamus where oxytocin is synthesized and secreted by the pituitary gland. The stimulation of the nuclei causes the release of oxytocin down the pituitary stalk and into the posterior pituitary gland, where oxytocin is stored.
-
Oxytocin is released when the infant's suckling creates afferent impulses that stimulate the posterior pituitary gland. It is released in a pulsatile fashion to adjacent capillaries, traveling to the mammary myoepithelial cell receptors that, when bound with oxytocin, stimulate the cells to contract. This contraction of the myoepithelial cells that line the ducts of the breast result in milk being expelled from the alveoli and into the ducts, which then empty through a nipple pore and into the infant’s mouth.
Milk secretion directly correlates with synthesis, and the regulation of milk synthesis is quite efficient. Milk synthesis remains remarkably constant at approximately 800 mL/d. However, the actual volume of milk secreted may be adjusted to the requirement of the infant by feedback inhibitor of lactation (FIL), a local factor that is secreted into the milk; therefore, the rate of milk synthesis is related to the degree of breast emptiness or fullness. The emptier breast produces milk faster than the fuller one.
Milk production is responsive to maternal states of well-being. Thus, stress and fatigue adversely affect a woman's milk supply. The mechanism for this effect is the downregulation of milk synthesis with increased levels of dopamine, norepinephrine, or both, which inhibit prolactin synthesis. Relaxation is key for successful lactation.
Biochemistry of human milk
Human milk is a unique, species-specific, complex nutritive fluid with immunologic and growth-promoting properties. This unique fluid actually evolves to meet the changing needs of the baby during growth and maturation. [14] Milk synthesis and secretion by the mammary gland involve numerous cellular pathways and processes (summarized in the figure below).
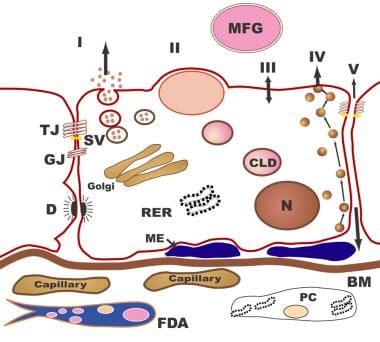 Human Milk and Lactation. The pathways for milk secretion and synthesis by the mammary epithelial cell. I: Exocytosis of milk protein, lactose, and other components of the aqueous phase in Golgi-derived secretory vesicles. II: Milk fat secretion via the milk fat globule. III: Direct movement of monovalent ions, water, and glucose across the apical membrane of the cell. IV: Transcytosis of components of the interstitial space. V: The paracellular pathway for plasma components and leukocytes. Pathway V is open only during pregnancy, involution, and in inflammatory states such as mastitis. BM = basement membrane; CLD = cytoplasmic lipid droplet; D = desmosome; FDA = fat-depleted adipocyte; GJ = gap junction; ME = myoepithelial cell; MFG = milk fat globule; N = nucleus; PC = plasma cell; RER = rough endoplasmic reticulum; SV = secretory vesicle; TJ = tight junction.
Human Milk and Lactation. The pathways for milk secretion and synthesis by the mammary epithelial cell. I: Exocytosis of milk protein, lactose, and other components of the aqueous phase in Golgi-derived secretory vesicles. II: Milk fat secretion via the milk fat globule. III: Direct movement of monovalent ions, water, and glucose across the apical membrane of the cell. IV: Transcytosis of components of the interstitial space. V: The paracellular pathway for plasma components and leukocytes. Pathway V is open only during pregnancy, involution, and in inflammatory states such as mastitis. BM = basement membrane; CLD = cytoplasmic lipid droplet; D = desmosome; FDA = fat-depleted adipocyte; GJ = gap junction; ME = myoepithelial cell; MFG = milk fat globule; N = nucleus; PC = plasma cell; RER = rough endoplasmic reticulum; SV = secretory vesicle; TJ = tight junction.
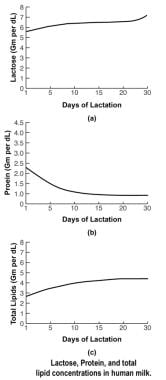
The processing and packaging of nutrients within human milk changes over time as the recipient infant matures. For example, early milk or colostrum has lower concentrations of fat than mature milk but higher concentrations of protein and minerals (see the image below). This relationship reverses as the infant matures.
Important biochemical points are discussed below.
In addition to the changes from colostrum to mature milk that mirror the needs of the developing neonate, variation exists within a given breastfeeding session. The milk first ingested by the infant (fore milk) has a lower fat content. As the infant continues to breastfeed over the next several minutes, the fat content increases. This hind milk is thought to facilitate satiety in the infant. Finally, the diurnal variations in breast milk reflect maternal diet and daily hormonal fluctuations.
Specific enzymes to aid neonatal digestion
Human milk contains various enzymes; some are specific for the biosynthesis of milk in the mammary gland (eg, lactose synthetase, fatty acid synthetase, thioesterase), whereas others are specific for the digestion of proteins, fats, and carbohydrates that facilitate the infant's ability to break down food and absorb human milk. Certain enzymes also serve as transport moieties for other substances, such as zinc, selenium, and magnesium.
Three-dimensional structure of human milk
Under a microscope, the appearance of human milk is truly amazing. Although it is a fluid, human milk has substantial structure in the form of compartmentation. Nutrients and bioactive substances are sequestered within various compartments. The most elegant example of this structure involves lipids, which are enveloped at the time of secretion from the apical mammary epithelial cell within its plasma membrane, becoming the milk-fat globule. Certain proteins, growth factors, and vitamins also become sequestered within this milk-fat globule and are embedded within the membrane itself.
The membrane acts as a stabilizing interface between the aqueous milk components and compartmentalized fat. This interface allows controlled release of the lipolysis products and transfer of polar materials into milk serum (aqueous phase). The bipolar characteristics of the membrane are also necessary for the emulsion stability of the globules themselves; thus, the structure of human milk provides readily available fatty acids and cholesterol for micellar absorption in the small intestine.
Proteins, carbohydrates, and designer fats for optimal brain development
Human milk provides appropriate amounts of proteins (primarily alpha-lactalbumin and whey), carbohydrates (lactose), minerals, vitamins, and fats for the growing term infant. The fats are composed of cholesterol, triglycerides, short-chain fatty acids, and long-chain polyunsaturated (LCP) fatty acids. The LCP fatty acids (18- to 22-carbon length) are needed for brain and retinal development. Large amounts of omega-6 and omega-3 LCP fatty acids, predominately the 20-carbon arachidonic acid (AA) and the 22-carbon docosahexaenoic acids (DHAs), are deposited in the developing brain and retina during prenatal and early postnatal growth.
An infant, particularly a preterm infant, may have a limited ability to synthesize optimal levels of AA and DHA from linoleic and linolenic acid. Therefore, these two fatty acids may be considered essential fatty acids. Many infant formulas in the United States have added AA, DHA, or both. The amount of AA and DHA in breast milk varies with the maternal diet. [5, 14, 15, 16] The unique blend of fatty acids in the breast milk has been linked to the development of innate and adaptive immune regulation.
Prior to routine fortification of formulas with DHA and AA, infants who received breast milk demonstrated better visual acuity at age 4 months than formula-fed infants, as well as had slightly enhanced cognitive development. [17] This has not been a universal finding, however, and some investigators have continued to doubt the benefits of DHA and AA. However, in a study of children aged 5 years who were breastfed and whose mothers were given a modest DHA supplement until 4 months postpartum, there was a significant improvement in sustained attention when compared to children whose mothers were not given DHA. [18]
Preterm formulas were also found to improve bone mineralization. Preterm formula given to very low birthweight (VLBW) preterm infants has been found to better aid growth and development than giving term formula to this population. [19]
A study that examined maternal dietary manipulation of fatty acid concentration and neurodevelopmental differences in human milk found no differences in neurodevelopmental outcomes despite higher levels of AA and DHA in the supplemented maternal group. [20] This finding supports a more global effect of human milk as opposed to a single agent that renders developmental differences.
Whether healthy term infants benefit from the addition of DHA and AA to formula remains unclear, because they are able to convert very LCP fatty acids to DHA and AA. Term infants who are ill and those born prematurely are most likely to benefit from formulas enriched with DHA, AA, or both.
Rather than producing better vision or greater intelligence, breast milk may somehow protect the developing neonatal brain from injury or less optimal development by providing necessary building materials and growth factors that act synergistically rather than in isolation.
A study by Dallas et al indicated that milk produced by women who deliver preterm demonstrates a high level of protein breakdown by endogenous proteases, with the investigators suggesting that such breakdown may reduce difficulties associated with the immature digestive systems of preterm infants. [21] The study, which looked at a total of 32 term and 28 preterm milk samples (from 8 mothers and 14 mothers, respectively), found preterm milk to have a significantly higher peptide count than term milk. Cleavage-site analysis suggested that the protease plasmin is more active in preterm milk and that cytosol aminopeptidase and carboxypeptidase B2 also break down milk proteins. [21]
Immunologic properties of human milk
Knowledge about the immune properties and effects of human milk continues to grow. Breast milk is a rich source of immunoglobulins, lactoferrin, lysozymes, cytokines, and numerous other immunologic factors that provide both active and passive immunity to the breastfeeding infant. A recommended comprehensive review by one of the pioneers in the field, Dr Armand Goldman, appeared in Breastfeeding Medicine (2007). [22] Below are the highlights of some many known immune properties and functions of human milk.
Human milk immunoglobulins
Human milk contains all of the different antibodies (M, A, D, G, E), but secretory immunoglobulin A (sIgA) is the most abundant. Milk-derived sIgA is a significant source of passively acquired immunity for the infant during the weeks before endogenous production of sIgA occurs. During this time of reduced neonatal gut immune function, the infant has limited defense against ingested pathogens. Therefore, sIgA is an important protective factor against infection. This is especially true for the preterm infant in whom the innate ability of the gut to produce its own sIgA is delayed beyond that of term infants and, thus, the predominant source of sIgA in this population is from maternal milk. [23]
Assuming that the mother and her infant, who are closely associated, share common flora, the antigenic specificity of the mother's sIgA in her milk is directed against the same antigens in the neonate. Maternal immunoglobulin A (IgA) antibodies derived from the gut and respiratory immune surveillance systems are transported via blood and lymphatic circulations to the mammary gland, and then ultimately to be extruded into her milk as sIgA. The packaging of IgA with a secretory component unique to the mammary gland protects sIgA from stomach acids in the infant, allowing it to reach the small intestine intact.
Other immunologic properties of human milk
In addition to antibodies, human milk has numerous other immunologic factors that interact with the gut microbiome to improve intestinal health. Lactoferrin is the major whey protein in human milk and plays a significant role in the innate response to infection with its diverse antimicrobial and anti-inflammatory properties. Lactoferrin also helps promote the growth of beneficial bacteria and reduce the colonization of pathogenic species. [24] One of the ways it can do this is by binding to iron, which then prevents the growth of various pathogens that rely on iron for further proliferation. Lactoferrin has also been shown to inhibit microbial adhesion to host cells and has direct cytotoxic effects against bacteria, viruses, and fungi, specifically by forming lactoferricin, a potent cationic peptide with bactericidal activity that is formed during digestion of lactoferrin. [25]
Human milk oligosaccharides (HMOs) are the third most abundant component of human breast milk after lactose and lipids. HMOs are believed to directly influence the gut microbiome by acting as a prebiotic for specific beneficial bacteria and reducing adhesion to gut epithelium by pathogenic bacteria. [25] The type and amount of the secreted HMOs in breast milk are genetically predetermined, influenced by maternal and other factors, and they are highly variable among mothers and across lactation stages. [26]
Human milk glycoproteins (HMGPs) are also an important immunologic component of human milk that have inhibitory activity against a broad spectrum of pathogens. One of the most widely recognized HMGPs are mucins. Mucins are major components of the extracellular matrix that are involved in diverse functions, including shielding the epithelium against pathogenic infection, regulating cellular signaling, and transcription. [27]
Our understanding of the interactional effect of these bioactive constituents, the impact of microbiota on gut function, and development (and role of human milk in that development) is just beginning to be understood. These constituents clearly have profound effects on the health status of individuals throughout life, particularly during infancy. [28, 29, 30, 23]
Human milk leukocytes
Maternal leukocytes from breast milk provide active immunity to the infant by fighting pathogens directly via phagocytosis, producing bioactive components, assisting in the development of the newborn immune system, or modifying the microenvironment of the infant digestive tract. [31] The stage of lactation is associated with major changes in milk leukocyte composition. In a study by Trend et al, the total amount of leukocytes and concentrations of the different subsets varied at different time points in lactation. Their results showed that colostrum contained approximately 146,000 cells/mL; the amount decreased in transitional (8-12 days postpartum) and mature milk (26-30 days postpartum) to 27,500 and 23,650 cells/mL, respectively. [32]
Passive immunity from mother to recipient breastfeeding infant
While awaiting endogenous maturation of the baby's own immunologic systems, various immunologic and bioactive milk components act synergistically to provide a passive immunologic support system from the mother to her infant in the first days to months after birth. [33] Ingested milk passively immunizes the neonate. Numerous studies have clearly documented this scenario and its clinical benefit, demonstrating a decreased risk for gastrointestinal and respiratory infections, particularly during the first year of life. [14, 22, 34]
Evidence is increasing that these immune and bioactive substances prime the neonatal gastrointestinal and immune systems in their selective recognition of antigens and development of cellular signaling. This may explain the decreased risk of intestinal and respiratory allergies in children who have been breastfed and the lower-than-predicted risk of autoimmune diseases in the breastfed population. Direct effects are difficult to prove given the multifactorial nature of such diseases; however, when taken together, the data support the beneficial nature of human milk for the developing infant.
Bioactive properties of human milk
Human milk also contains growth modulators, such as epidermal growth factor (EGF), nerve growth factor (NGF), insulinlike growth factors (IGFs), and interleukins (ILs). Transforming growth factor (TGF)–alpha, TGF-beta, and granulocyte colony-stimulating factor (G-CSF) are also identified in human milk. These growth modulators are produced either by the epithelial cells of the mammary gland or by activated macrophages, lymphocytes (mainly T cells), or neutrophils in the milk. EGF and TGF-alpha has been found at higher concentrations in the milk of mothers who delivered prematurely compared to those who delivered at term. EGF is important in gut epithelial regeneration and repair, which may explain why maternal milk has been shown to reduce the risk for necrotizing enterocolitis. [35] EGF, TGF-alpha, and human milk stimulate fetal small intestinal cell proliferation in vitro, with the greatest increase in cell proliferation seen following exposure to human milk.
Certain bioactive substances and live cells in human milk appear to influence neonatal gut maturation and growth through their transfer of developmental information to the newborn. Although most of these biosubstances have been identified in quantities that exceed maternal serum levels, their exact role in human newborns is uncertain; most current information is from animal models whose development may significantly differ from that of a human infant.
Human Milk and Preterm Infant
Human milk feeding has several life-long, important beneficial health effects, in a dose-dependent relation; its promotion and support should be considered as a public health issue for the benefit of population at large. [36] However, keep in mind that breastfeeding initiation and duration are more challenging in preterm infants, [37] and that premature infant's mother's milk is qualitatively very different from the term infant's mother's milk.
Donor human milk has been studied for its role as a mother’s milk substitute. Donor milk has to be processed through pasteurization for microbiologic safety reasons, and it is supplemented with fortifiers. Nonetheless, it is a better feeding alternative for preterm infants relative to formula when the mother's own milk is not available. [1] The processing of donor milk has a variable impact on the concentration of vitamins, enzymes, and nutrients, resulting in a diminished bioactive function of donor milk. [38] Innovative techniques including high-pressure processing have been used to study the potential for qualitative changes to donor human milk compounds, such as changes in concentrations after pasteurization of water-soluble forms of choline, which is crucial for infants’ development. [39, 40]
Despite the alterations in donor milk secondary to processing, relatively recent data suggest that providing donor milk to extremely premature infants reduces the risk of necrotizing enterocolitis (NEC) when compared to formula-fed preterm infants. However, formula-fed infants have better weight gain, linear growth, and head growth when compared to those fed donor milk. [41] Despite the slower growth with donor milk, when a mother's own milk is not available, donor milk should be considered.
More recently, evidence suggests that preterm infants fed either maternal breast milk or banked donor breast milk not only have a reduced risk of neonatal infection and NEC but, as a consequence of the higher nutrient diet and prevention of neonatal infection/NEC, these infants may also have improved long-term cognitive outcome in childhood, such as a higher verbal intelligence quotient. [42]
Conclusion
Human milk, in addition to its numerous nutrients that make it an ideal food source for the growing term infant, is a bioactive fluid that evolves from colostrum to mature milk as the infant matures. This bioactive fluid contains numerous factors and live cells that, in concert, promote the growth and well-being of the breastfeeding infant. Oliver Wendell Holmes said it best when he stated, "A pair of substantial mammary glands has the advantage over the two hemispheres of the most learned professor's brain, in the art of compounding a nutritious fluid for infants." [43] With the ever-expanding knowledge resulting from research, commercial formula clearly cannot replicate all of the valuable properties that are inherent in human milk.
-
Human Milk and Lactation. Schematic diagram of the breast.
-
Human Milk and Lactation. Frontal view of lactating breast.
-
Human Milk and Lactation. Myoepithelial cells, open and contracting.
-
Human Milk and Lactation. The pathways for milk secretion and synthesis by the mammary epithelial cell. I: Exocytosis of milk protein, lactose, and other components of the aqueous phase in Golgi-derived secretory vesicles. II: Milk fat secretion via the milk fat globule. III: Direct movement of monovalent ions, water, and glucose across the apical membrane of the cell. IV: Transcytosis of components of the interstitial space. V: The paracellular pathway for plasma components and leukocytes. Pathway V is open only during pregnancy, involution, and in inflammatory states such as mastitis. BM = basement membrane; CLD = cytoplasmic lipid droplet; D = desmosome; FDA = fat-depleted adipocyte; GJ = gap junction; ME = myoepithelial cell; MFG = milk fat globule; N = nucleus; PC = plasma cell; RER = rough endoplasmic reticulum; SV = secretory vesicle; TJ = tight junction.
-
Human Milk and Lactation. Lactose, protein, and total lipid concentrations in human milk.
-
Human Milk and Lactation. (A) Ultrasound image of milk duct in the lactating breast. The duct appears as a branching hypoechoic structure within echogenic glandular tissue. (B) The ducts focused on the nipple (N) to the periphery of the breast. The walls are echogenic (up arrow) and the lumen hypoechoic (asterisk). The first branch of this duct (-->) is imaged almost directly under the nipple.