Overview
This article describes important principles and specific methods of fluid, electrolyte, and nutrition (FEN) management in newborns, with a special focus on patients with complex fluid and electrolyte requirements. These include premature newborns with very low birth weight (VLBW) and extremely low birth weight (ELBW), as well as infants who have undergone abdominal surgery and those who have sepsis. (See the image below).
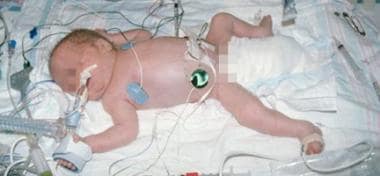 An ill preterm infant, such as this patient, requires fluid, electrolyte, and nutrition management in a neonatal intensive care unit.
An ill preterm infant, such as this patient, requires fluid, electrolyte, and nutrition management in a neonatal intensive care unit.
Fluid, electrolyte, and nutrition management in the context of acid-base disorders (eg, acidosis, alkalosis), hypercalcemia, magnesium disorders, metabolic disorders, and complications of total parenteral nutrition (TPN) are not discussed in this article.
Fluid, electrolyte, and nutrition management is important because most infants in a neonatal intensive care unit (NICU) require intravenous fluids (IVFs) and have shifts of fluids between intracellular, extracellular, and vascular compartments. Therefore, careful attention to fluid and electrolyte balance is essential. If inappropriate fluids are administered, serious morbidity may result from fluid and electrolyte imbalances. Inadequate attention to nutrition in the neonatal period leads to growth failure, osteopenia of prematurity, and other complications.
Prevalence and Loss of Body Water
Principles of fluid and electrolyte balancing include the following:
-
Total body water (TBW) equals intracellular fluid (ICF) plus extracellular fluid (ECF)
-
ECF equals intravascular fluid (plasma and lymph in the vessels) plus interstitial fluid (between cells)
Body water composition
The percentage of the body composed of water is higher for a term neonate than it is for an adult, with a newborn being 75% water (40% ECF, 35% ICF) and an adult being 60% water (20% ECF, 40% ICF). Term newborns usually lose 5-10% of their weight in the first week of life, almost all of which is water loss.
Preterm neonates have proportionally more water (at 23 weeks' gestation, the body is 90% water; 60% ECF and 30% ICF), and they may lose 10-15% of their weight in the first week of life. Small for gestational age (SGA) preterm infants may also have a particularly high body water content (90% for SGA infants vs 84% for appropriate for gestational age [AGA] infants at 25-30 weeks’ gestation). [1]
Insensible water loss
Insensible water loss (IWL) is water loss that is not readily measured. It consists mostly of water lost via evaporation through the skin (two thirds) or respiratory tract (one third).
IWL varies with gestational age; the earlier the gestational age of the preterm infant, the greater the IWL. Evidence from animal studies suggests that aquaporin channels that regulate IWL are developmentally regulated. [2]
The magnitude of IWL also depends on the postnatal age. Because skin thickens with age, the IWL decreases as premature neonates mature. [3] Ventilated infants receive humidified gas. Therefore, IWL from the lungs is eliminated in these infants.
Sensible water loss
Other measurable sources of fluid loss include urine, stool (eg, diarrhea and ostomy), nasogastric (NG) or orogastric (OG) drainage, and cerebrospinal fluid (CSF) loss (eg, ventricular drainage).
Renal function changes
Neonates have a decreased capacity to concentrate or dilute urine in response to changes in intravascular fluid status and are at risk for dehydration or fluid overload. The normal maturation of renal function that occurs with increasing gestational and postnatal age also plays a role in determining fluid requirements.
Assessing Fluid and Electrolyte Status
Numerous conditions can affect neonatal fluid and electrolyte balance, as well as renal function. The presence of several of these can be suspected on the basis of information found during the prenatal and neonatal history.
Maternal history
A newborn's fluid and electrolyte status partially reflects the mother's status. For example, excessive administration of oxytocin or hypotonic IVF to the mother can cause hyponatremia in the neonate at birth.
Placental dysfunction (eg, due to hypertension in pregnancy) can adversely affect intrauterine growth. Infants who exhibit growth retardation at birth (< 10th percentile for gestational age) may grow poorly unless their nutritional needs are specifically addressed after birth. The severity and duration of the poor intrauterine malnutrition influences the degree of postnatal catch-up growth.
Poorly controlled maternal diabetes may be associated with renal vein thrombosis. This can adversely affect an infant's renal function.
Maternal use of angiotensin-converting enzyme (ACE) inhibitors, such as captopril, during pregnancy can lead to acute renal failure in infants. Other medications administered to the mother, including indomethacin, furosemide, and aminoglycoside, may also affect renal function in the neonate.
Antenatal steroids may increase skin maturation, thereby decreasing IWL and the risk of hyperkalemia. [4, 5]
Newborn history
The presence of oligohydramnios may be associated with congenital renal dysfunction, such as renal agenesis, polycystic kidney disease, or posterior urethral valves. Severe in utero hypoxemia or birth asphyxia may lead to acute tubular necrosis.
In the infant, posterior urethral valves can be suspected when spontaneous urination is lacking or when a weak urinary stream and dribbling are present. Frequently, the bladder is full.
The environment in which an infant is cared for affects fluid loss. An environment with high ambient humidity decreases IWL, whereas the use of a radiant warmer or phototherapy may significantly increase an infant's IWL. In infants who are intubated, inadequate humidification of the inspired gas may also lead to increased IWL.
For numerous reasons, an infant's weight and extracellular water volume may significantly increase while intravascular volume decreases. For example, peritonitis or the long-term use of paralytic agents can lead to increased interstitial fluid volume, increased fluid in the bowel and peritoneal cavity, and increased body weight with decreased intravascular volume. This is sometimes referred to as fluid in the third space or third spacing of fluid.
Clinical evaluation of fluid status
An inability to keep a baby sufficiency warm can signal the occurrence of excessive IWL. Because of the latent heat of evaporation, cooling occurs with IWL, similar to cooling due to sweating in older children and adults.
Sudden changes in an infant's weight generally reflect a change in body water. The compartment affected depends on the gestational age and associated problems (eg, respiratory distress syndrome, sepsis, necrotizing enterocolitis) and clinical care.
Histologic and mucosal manifestations are also noted in water loss. However, altered skin turgor, a sunken anterior fontanelle (AF), and dry mucous membranes are not sensitive indicators of dehydration in babies. Remembering that premature infants have poorly keratinized skin that leads to a marked elevation in IWL is important.
Although growth charts are valuable in following growth parameters and nutritional status over time, they play little role in the daily management of fluid and electrolyte balances.
Cardiovascular symptoms
Tachycardia may be a reflection of decreased intravascular volume, decreased stroke volume, or ineffective cardiac output. This may be independent of the status of the ECF volume, which is increased in congestive heart failure and decreased in dehydration.
Although delayed capillary refill occurs in low cardiac output states, it can also be seen in infants with peripheral vasoconstriction that results from cold stress or acidosis.
As a result of an infant's compensatory mechanisms, blood pressure (BP) readings are usually within the reference range with mild or moderate hypovolemia. With severe hypovolemia, hypotension is almost invariably present.
Laboratory evaluation
Depending on the clinical situation and the suspected etiology of fluid and electrolyte derangements, some or all of the tests below may be warranted.
Serum electrolyte, urea nitrogen, creatinine, and plasma osmolarity levels should be assessed. Keep in mind that results of these tests, especially the creatinine levels, may still reflect maternal values over the first 12-24 hours. Serum creatinine normally declines postnatally, but very premature infants may have a delay in the decrease of serum creatinine levels. [6]
Accurate total urine output and total fluid intake may be assessed. In infants without urinary catheters or urine bags, diapers need to be weighed soon after voiding to reduce errors due to evaporation. [7] Infants with reduced urine output and a serum creatinine that does not decline postnatally or increases may have acute kidney injury (AKI).
Urine electrolyte levels and specific gravity may be assessed. If the infant is being treated with diuretics, such as furosemide, results of these tests are difficult to interpret.
Calculation of the fractional urinary excretion of sodium in relation to creatinine (FENa) and blood gas analysis may be indicated; metabolic acidosis may be a marker of inadequate tissue perfusion.
Fluid and Electrolyte Management
Management goals
Fluid and electrolyte management is achieved with constant assessment of fluid intake and output, as well as monitoring of basic laboratory chemistries. Primary goals are to maintain the appropriate ECF volume, ECF and ICF osmolality, and ionic concentrations.
The initial loss of ECF over the first week must be allowed, as reflected by weight loss, while maintaining normal intravascular volume and tonicity, as reflected by heart rate, urine output, and electrolyte and pH values. Subsequently, maintain water and electrolyte balance while supplying requirements for body growth. The clinical approach must be individualized, relying on norms for gestational age and birth weight for guidance.
Total fluid requirements
Total fluid requirements equal maintenance requirements (IWL plus urine plus stool water) plus growth requirements. In the first few days, IWL is the largest component of lost fluids. Later, as the renal solute load increases, the amount of water the kidneys need to excrete this load increases (80-120cal/kg/day equal 15-20mOsm/kg/day, which means that 60-80mL/kg/day are needed to excrete wastes). Stool requirement is usually 5-10mL/kg/d. As infants add tissue, they also need to add water to maintain normal ECF and ICF volumes. Because weight gain is 70% water, an infant growing 30-40g/day requires an additional 20-25mL/day of water.
Factors that modify fluid requirements
As the skin matures postnatally, the IWL decreases. Elevated body and environmental temperatures increase IWL. Radiant warmers increase IWL by 50%, phototherapy may increase IWL, and the use of a plastic heat shield reduces IWL by 10-30%. Environmental humidification decreases IWL from the skin and respiratory mucosa by as much as 30%.
Skin breakdown and skin defects (eg, omphalocele) proportionally increase IWL to the affected area. Infants exposed to antenatal steroids have lower IWL as well as better diuresis. [8]
Electrolyte requirements
For the first 24 hours, supplemental sodium, potassium, and chloride are not usually required. Starting at age 24 hours, assuming that urine production is adequate, the infant needs 1-2mEq/kg/day of potassium and 1-3 mEq/kg/day of sodium.
Extremely premature infants who develop metabolic acidosis may benefit from sodium acetate administration instead of sodium chloride. [9] Some evidence suggests that metabolic acidosis in preterm infants is primarily due to inadequate urinary acidification by NH4+ excretion and loss of bicarbonate. [10]
During the active growth period after the first week, the need for potassium may increase to 2-3mEq/kg/day, and the need for sodium and chloride may increase to 3-5mEq/kg/day. Some of the smallest preterm infants have sodium requirements of as much as 6-8mEq/kg/day because of the decreased capacity of the kidneys to retain sodium.
Fluids and Electrolytes in Common Neonatal Conditions
Infants with respiratory distress syndrome need appropriate fluid replacement. Administration of excessive fluid, however, can lead to hyponatremia and volume overload, worsening the pulmonary condition and increasing the risk that bronchopulmonary dysplasia (BPD) will develop. Inadequate fluid administration leads to hypernatremia and dehydration.
As a result of increased work in breathing, infants with BPD have higher energy requirements. Diuretics are often prescribed in these infants to treat pulmonary edema, which can lead to electrolyte disturbances.
Avoiding excessive fluid administration is critical in infants with a patent ductus arteriosus (PDA) because this often worsens their respiratory status. This is especially important when indomethacin is prescribed to treat PDA, because indomethacin can decrease urine output and, in fact, require restriction of fluid administration.
Infants who have experienced perinatal asphyxia may have involvement of multiple organ systems. They are prone to acute tubular necrosis and significant oliguria, and central nervous system (CNS) injury may produce syndrome of inappropriate antidiuretic hormone secretion (SIADH). Restricting fluid intake to minimize the risk of volume overload is often required. However, no evidence from randomized, controlled trials indicates that this practice reduces morbidity or mortality. [11]
Common Electrolyte Problems
Hyponatremia
Hyponatremia is defined as a serum sodium level of less than 130mEq/L. Usually, this is not a cause for concern until the serum sodium has dropped to less than 125mEq/L. Remember that hyponatremia usually results from excessive free water intake relative to insensible and sensible water loss. However, inadequate sodium intake can contribute to the development of hyponatremia, especially in the extremely premature infant with increased sodium loss.
Hypernatremia
Hypernatremia is defined as a serum sodium level greater than 150mEq/L. Usually, this is not a cause for concern until the serum sodium level has risen to greater than 155mEq/L. Hypernatremia is commonly seen in the first few days of life in ELBW preterm infants and most often occurs when free-water intake is inadequate to compensate for very high IWL.
Very rarely, hypernatremia is the result of excessive administration of sodium in either the diet or IVFs. A common cause of excessive administration of sodium is associated with the administration of sodium bicarbonate to infants with pulmonary hypertension or metabolic acidosis in an effort to increase blood pH levels.
Remember that most of the potassium in the body is contained in the intracellular compartment; therefore, serum potassium levels often do not accurately indicate total-body potassium stores.
Hypokalemia
Serum potassium levels also depend on blood pH levels because pH affects the distribution of potassium between ICF and ECF compartments. A low pH level shifts K+ out of the cell, whereas alkalosis drives K+ into the cell. Therefore, acidosis increases the potassium concentration in the blood or serum, whereas alkalosis lowers the potassium concentration. A handy rule is that 0.1 U of pH change results in a 0.3-0.6mEq/L change in the serum potassium level.
Hypokalemia is defined as a serum potassium level of less than 3.5mEq/L. Unless the patient is receiving digoxin therapy, hypokalemia is rarely a cause for concern until the serum potassium level is less than 3.0mEq/L. Hypokalemia often results from chronic diuretic use and unreplaced electrolyte loss from NG drainage. Electrocardiographic manifestations of hypokalemia include a flattened T wave, prolongation of the QT interval, or the appearance of U waves.
Severe hypokalemia can produce cardiac arrhythmias, ileus, and lethargy. When significant, this condition is treated by slowly replacing potassium either intravenously or orally. Rapid administration of potassium chloride is not recommended, because it is associated with life-threatening cardiac dysfunction.
Hyperkalemia
Hyperkalemia is defined as a serum potassium level of greater than 6mEq/L measured in a nonhemolyzed specimen. Hyperkalemia is of far more concern than hypokalemia, especially when serum potassium levels exceed 6.5 mEq/L or if electrocardiographic changes have developed. Electrocardiographic manifestations of hyperkalemia are a progression from peaked T waves, as the earliest sign, to a widened QRS configuration, bradycardia, tachycardia, supraventricular tachycardia (SVT), ventricular tachycardia, and ventricular fibrillation.
Causes of hyperkalemia include potassium release from damaged neuronal cells and breakdown of red blood cells (RBCs) following intraventricular hemorrhage (IVH), trauma, and intravenous hemolysis. (See the image below.)
In addition, severe acidosis and decreased urinary potassium excretion contribute to elevations in serum potassium. Finally, hyperkalemia may be one of the earliest manifestations of congenital adrenal hyperplasia.
Management of significant hyperkalemia consists of various measures. All administration of potassium is discontinued. Calcium gluconate 100-200mg/kg (1-2mL/kg of 10% solution) is administered as a slow intravenous (IV) infusion over 5-10 minutes. Alkalinization is performed, either with hyperventilation or IV sodium bicarbonate 1-2mEq/kg. Inhaled albuterol enhances cellular uptake of potassium. Insulin is administered to assist in driving potassium into the ICF compartment. Insulin must be administered with glucose as a combined infusion to avoid hypoglycemia.
Medications are administered to enhance potassium excretion, including IV furosemide 1mg/kg or rectally administered sodium polystyrene sulfonate (Kayexalate) 1g/kg (do not use sorbitol-containing products and do not administer orally). Several hours must pass before any effect is observed with either of these medications. Dialysis or exchange transfusion may be used to assist in more rapidly removing potassium from the body.
Hypercalcemia and hypocalcemia
Total serum calcium levels in term infants decline from values of 10-11mg/dL at birth to 7.5-8.5mg/dL over the first 2-3 days of life. Approximately 50% of the total calcium is in the ionized form and is the only biologically available form of calcium. Ionized calcium values, rather than total values, correlate better with calcium functions, such as cardiac contractility. Therefore, many centers rely exclusively on measurements of ionized calcium.
Calcium concentrations can be reported either in milligrams per deciliter (mg/dL) or in millimolar units (mmol/L). Conversion between the 2 methods is accomplished by dividing by 4 (eg, 4mg/dL of ionized calcium equal 1mmol/L).
Hypercalcemia is rarely observed in neonates; it is defined as a total serum calcium concentration of higher than 11mg/dL or an ionized calcium concentration of higher than 5mg/dL (1.25mmol/L).
Hypocalcemia is more common and is defined as a total serum calcium concentration of less than 7mg/dL or an ionized calcium concentration of less than 4mg/dL (1mmol/L).
Early onset hypocalcemia may occur within the first 3 days of life in premature infants born to mothers with poorly controlled diabetes or in infants who experienced perinatal asphyxia. If the infant is asymptomatic and has a total serum calcium level of more than 6.5mg/dL or an ionized calcium level of more than 0.8-0.9mmol/L, close observation alone is appropriate. Calcium supplementation should be provided if the total serum calcium level is less than 6.5mg/dL or if the ionized level is less than 0.8-0.9mmol/L.
Late-onset hypocalcemia develops after the first week of life and is usually associated with conditions with high serum phosphate levels, including hypoparathyroidism, maternal anticonvulsant use, and vitamin D deficiency. Vitamin D deficiency usually resolves with reduction of the renal phosphate load or with vitamin D supplementation.
Oliguria
Oliguria, a common fluid problem, is defined as a urine output of less than 1mL/kg/h. Oliguria can be caused by various conditions that can be classified as prerenal, renal, or postrenal problems. Urine output is often less than 1mL/kg/h during the first 12-18 hours after birth. Most healthy term babies urinate within the first 12 hours; however, a small number of healthy infants may not urinate until 24-36 hours after birth. Persistent oliguria beyond 36 hours should be evaluated in an otherwise healthy infant.
Nutritional Requirements
Nutrient requirements include the following:
-
Energy (measured as cal/kg/day)
-
Carbohydrates
-
Water
-
Minerals and trace elements
-
Protein
-
Vitamins
-
Fat
Energy needs
The exact energy needs of a given neonate depend on several factors, including gestational age, postnatal age, weight, route of energy intake, growth rate, activity, and thermal environment. Infants who are ill or experiencing stressful situations (sepsis, surgery, BPD) have higher energy requirements.
Infants who receive parenteral nutrition need less energy for adequate growth because they do not need to absorb the provided nutrients and have no fecal losses. As a result, 70-90cal/kg/day with 3-3.5g/kg/day of protein may be adequate for growth.
Protein is not an ideal source of energy; rather, it is provided as a building block for new tissue. If adequate nonprotein calories are provided, the nitrogen balance in the infant is positive and the protein provided is used to build new tissue. Therefore, even if energy intake from protein is included in calculations of total energy intake, not all of the protein-derived calories are available for energy expenditure.
The ideal energy ratio provides 65% of the energy as carbohydrates and 35% as lipids. Most infants need 100-120cal/kg/day for adequate growth. Some need up to 160-180cal/kg/day (eg, infants with BPD).
The total energy needs of a growing, enterally fed premature infant without any acute illness are listed as follows:
-
Resting expenditure - 50cal/kg/day
-
Minimal activity - 4-5cal/kg/day
-
Occasional cold stress - 10cal/kg/day
-
Fecal loss (10-15% of intake) - 15cal/kg/day
-
Growth (4.5 cal/g of growth) - 45cal/kg/day
-
Total required to produce a 10g/day weight gain - 125cal/kg/day
Guidelines
The clinical practice guidelines on vitamin K prophylaxis in newborns were released in October 2018 by the Canadian Paediatric Society and the College of Family Physicians of Canada. [12]
Routine administration of vitamin K to newborns continues to be recommended by the Canadian Paediatric Society to prevent vitamin K–deficiency bleeding (VKDB). Intramuscular (IM) injection is preferred over oral (PO) administration.
The best-practice recommendation is that all newborns routinely receive one IM dose of vitamin K within the first 6 hours after birth, after initial stabilization and appropriate newborn/mother interaction. The dose is 0.5 mg for infants weighing 1500 g or less and 1 mg for infants weighing more than 1500 g.
Also recommended is to implement strategies to minimize the pain associated with IM injections.
Counsel parents who decline the IM injection about the serious health risks of VKDB. If the parents continue to decline, recommend PO vitamin K (2 mg) at the first feeding; this should be repeated at age 2-4 weeks and at age 6-8 weeks.
Advise parents that (1) IM vitamin K is more effective than PO vitamin K, (2) it is important all follow-up PO doses be received, and (3) the infant will remain at risk of developing late VKDB, with the potential to develop intracranial hemorrhage.
In preterm infants under intensive care, there is insufficient evidence to recommend intravenous vitamin K.
For more Clinical Practice Guidelines, please go to Guidelines.
Total Parenteral Nutrition
Goals for nutrition management
The primary goal in total parenteral nutrition (TPN) is to provide energy and nutrients in sufficient quantities to allow normal growth and development. [13] Although the goal is to have growth rates that follow either the intrauterine growth curve for premature infants or the postnatal growth curve for term infants, this is rarely achieved during the acute phase of an infant's illness. (See the image below.)
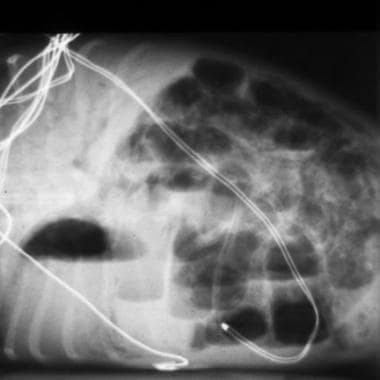 Radiograph depicts necrotizing enterocolitis in a preterm infant. Note the extensive pneumatosis intestinalis and the portal venous air. This situation often requires long-term administration of total parenteral nutrition.
Radiograph depicts necrotizing enterocolitis in a preterm infant. Note the extensive pneumatosis intestinalis and the portal venous air. This situation often requires long-term administration of total parenteral nutrition.
Calculations
When calculating FEN requirements, most practitioners use an infant's birth weight until the infant has regained the birth weight. Thereafter, daily weight is used in calculations. TPN can be started on the first day of life in infants who are not likely to achieve total enteral nutrition within the first week of life. Especially in infants who are ill, protein is required to decrease or prevent catabolism, and starting TPN on the first day is important. A standardized TPN solution (based on postnatal age) may be more convenient and cost-effective compared with individualized TPN. [14] The goal for TPN is to provide 378-420kJ/kg/day, with 3-3.5g/kg/day of protein. One kcal equals 4.1868kJ.
Calculate the infant's daily fluid (water) requirement. Calculate the amount of energy required. Determine the specific amounts and sources of carbohydrates and lipids. In addition, determine the amounts of vitamins and trace elements to deliver.
Determine the amount of protein to deliver based on the total number of calories to be provided. Remember that an infant needs an adequate number of nonprotein calories (630-840kJ/g nitrogen) to have a positive nitrogen balance. Most practitioners start at 1.5g/kg/day of protein on the first or second day and increase this by 0.5-1.0g/kg/day, as tolerated. Various amino acid preparations are commercially available for use in the neonate (eg, TrophAmine).
Carbohydrate
IV dextrose provides most of the energy in TPN. The caloric content of aqueous dextrose is 14.28kJ/g of glucose, which is equal to 142.8kJ/100mL of D10W. As a result of the high osmolarity of concentrated dextrose solutions, the maximum dextrose concentration that can be delivered safely through a peripheral vein is 12.5%. With central venous access, a dextrose concentration up to 15% is often used, and in special situations (eg, when fluids need to be restricted), a concentration of as much as 25% may be used.
A glucose infusion rate expressed in milligrams of glucose/kg/min is the most appropriate way to express glucose administration because the rate accounts for the glucose concentration and the rate of infusion.
Very small premature infants who weigh less than 1500g demonstrate impaired glucose tolerance. For this reason, in infants who weigh less than 1kg, start at an infusion rate of 6mg/kg/min. In infants who weigh 1-1.5kg, start at 8mg/kg/min. If the glucose infusion rate is excessive, hyperglycemia may develop. If blood glucose levels are greater than 150-180mg/dL, glucosuria may occur, which may lead to osmotic diuresis. This can be controlled by either decreasing the glucose infusion rate or treating the infant with insulin. Persistent hyperglycemia may need a continuous infusion of insulin. [15] Note that acute increases in the blood or serum glucose concentration when the glucose infusion rate is unchanged are often the first sign of sepsis in the preterm infant.
Fat
At least 3% of the total energy should be supplied as essential fatty acids (EFA). This can be accomplished by providing a fat emulsion (eg, Intralipid, Liposyn), 0.5g/kg/day 3 times per week. Fat emulsions provide about 37.8-42kJ/g.
Parenteral fat emulsion is usually provided as a 20% lipid emulsion made from soybeans (eg, Intralipid). Intralipid is a concentrated source of energy with a caloric density of 8.4kJ/mL (for 20% Intralipid). Lipids play a primary role in supporting gluconeogenesis in parenterally fed preterm infants. [16] Most practitioners start with 0.5-1.5g/kg/day on the first day and increase steadily to 3-3.5g/kg/day.
Limiting Intralipid infusions in infants with sepsis and severe lung disease is often recommended, although no strong, recent evidence supports this practice. The use of Intralipid (as well as prolonged TPN and use of central venous lines) is a risk factor for candidemia in neonates. [17]
Neonates with hyperbilirubinemia who are on phototherapy often have Intralipid intake restricted to less than 2g/kg/day (especially if bilirubin levels are rising while the infant is on phototherapy) because some evidence suggests that a high lipid emulsion intake may decrease bilirubin binding. [18] Many practitioners monitor triglyceride levels and adjust infusion rates to maintain triglyceride levels of less than 150mg/dL.
Infants with cholestasis (increase in conjugated bilirubin >2 mg/dL) due to parenteral nutrition (parenteral nutrition–associated liver disease [PNALD]) should preferably have their intralipid infusion reduced (eg, to 1 g/kg/day, given over 12 h). Some evidence suggests that use of lipid infusions with omega-3 fatty acids (eg, Omegaven) may reduce cholestasis, but further studies are currently required.
Protein
Term infants need 1.8-2.2g/kg/day along with adequate nonprotein energy for growth. Preterm VLBW infants need 3-3.5g/kg/day along with adequate nonprotein energy for growth. Usually, providing more than 4g/kg/day of protein is not advisable. Infants under stress or who have cholestasis are usually limited to 2.5g/kg/day of protein because the severity of TPN-induced cholestasis may depend on the duration of TPN and the amount of amino acids infused. [19, 20]
Protein administration should be started on the first day of life or as soon as fluid and electrolyte requirements have stabilized. Maintain a nonprotein-to-protein calorie ratio of at least 25-30:1. The current role of supplements, such as additional inositol and carnitine, is under investigation. Although a physiologic rationale for their use has been suggested, they have not yet been shown to be of benefit in large, randomized, controlled trials. [21, 22] The addition of glutamine has not been shown to improve outcome. [23]
Minerals (other than sodium, potassium, chloride)
Once protein intake has been started, calcium and phosphorous should be added to TPN. Calcium and phosphorous need to be concurrently administered for proper accretion. Take care to ensure that solubility is not exceeded; if this happens, calcium and phosphorous may spontaneously precipitate. Supplemental magnesium should be added to TPN once protein has been added.
Vitamins and trace elements
Vitamins A, D, E, and K are fat soluble. Vitamins B-1, B-2, B-6, B-12, C, biotin, niacin, pantothenate, and folic acid are water soluble.
Vitamin supplementation should be started as soon as protein is added to TPN. The addition of a commercially available neonatal vitamin preparation provides appropriate quantities of all vitamins, except possibly vitamin A. Vitamin A supplementation in ELBW infants has been shown to reduce death and BPD. [24] The usual dose of vitamin A is 5000 IU intramuscularly administered 3 times per week for the first 4 weeks in ELBW infants who receive respiratory support at age 24 hours.
The trace elements zinc, copper, selenium, chromium, manganese, molybdenum, and iodine also should be added to TPN once protein is started. This can be easily accomplished by the addition of a commercially available solution containing trace elements.
Enteral Nutrition
Energy
With enteral nutrition, human milk and standard infant formulas (20cal/oz) provide 67cal/100mL. In general, human milk is the preferred source of enteral nutrition because of its trophic and immunologic properties. Evidence is substantial that necrotizing enterocolitis is lower in preterm infants fed with breast milk.
Higher caloric density formulas include 22cal/oz, 24cal/oz, and 30cal/oz. Formulas with caloric densities higher than 24cal/oz should be used with caution because they may have a higher renal solute load and may not provide sufficient free water.
Carbohydrate
Lactose is the carbohydrate source in human milk and in most standard formulas given to term infants. Lactose provides 40-45% of the energy.
In preterm infant formulas, lactose provides 50% of the carbohydrates and glucose polymers provide 50%. This is because of the lower intestinal lactase levels and relatively higher intestinal glycosidase levels in premature infants. The use of glucose polymers (rather than monosaccharides or disaccharides) also helps to maintain a lower osmolality.
Soy and lactose-free formulas use sucrose, maltodextrins, and glucose polymers as the carbohydrate sources.
Fat
With enteral nutrition, approximately 50% of the energy is derived from fat. If more than 60% of the energy is derived from fat, ketosis is a risk.
Medium-chain triglycerides can be absorbed without pancreatic lipase or bile salt emulsification. Therefore, preterm infant formulas have a higher percentage of fat supplied as medium-chain triglycerides.
Protein
Protein requirements of 1.8-2.2g/kg/day are readily provided to term infants by human milk and standard infant formulas.
Preterm infant formulas have a higher protein content (3-3.5g/kg/day), in order to meet the growth requirements in this group of infants. If breast milk is used to provide enteral nutrition, then it should be blended with an appropriate fortifier so that it meets the protein needs of the preterm infant.
Minerals, vitamins, and trace elements
During the third trimester, accretion rates for calcium (120-150mg/kg/d) and phosphorous (75-85mg/kg/d) are higher than rates that can be provided in premature infants receiving human milk. As a result, a human milk fortifier is essential, and premature infants fed human milk must receive supplementation to minimize the risk of osteopenia of prematurity.
Premature infant formulas have a much higher concentration of these minerals, which helps approximate the third trimester accretion rates in infants receiving these formulas.
Human milk and term and premature infant formulas all provide amounts of magnesium adequate to meeting an infant's nutritional requirements if the infant is receiving at least 100cal/kg/day.
To minimize the risk of iron deficiency anemia, all formula-fed term infants should receive iron fortified formulas. Breastfed term infants should receive supplemental iron beginning when they are aged several months.
Premature infants should be started on supplemental iron once they receive full enteral feedings if they are fed human milk. If they are fed a premature infant formula, the issue of supplementation heavily depends on the amount of iron in the formula.
Full-term infants fed standard infant formula do not routinely require vitamin supplements, because adequate quantities of all of the vitamins are present in the formula.
Full-term infants fed human milk should receive supplemental vitamin D to minimize the risk of osteopenia and rickets. This may be provided by provision of a standard multivitamin supplement.
Premature infants fed human milk without human milk fortifier should be started on a multivitamin supplement as soon as they are receiving full enteral nutrition.
Premature infants receiving human milk with human milk fortifier or standard premature infant formulas should not routinely require additional vitamin supplements.
Special formulas
Numerous special infant formulas are available to meet the very specific dietary needs of small groups of patients who cannot be maintained on standard term or premature infant formulas. These formulas include soy-based formulas, elemental formulas, and formulas with unique protein, fat, and carbohydrate content.
As a result of the low calcium and phosphorous contents of soy-based formulas, they are not appropriate for premature infants unless supplementary calcium, phosphorus, or both are added to the formula. Specialty formulas are available for infants with galactosemia, phenylketonuria, short gut syndrome, and protein allergy, as well as many other conditions.
Enteral Feeding Methods
Premature infants in the NICU are usually fed by OG or NG tube until they are sufficiently mature to coordinate sucking, swallowing, and breathing. Then, the transition is made gradually to feeding by mouth (PO) at the breast or bottle. The staff at many NICUs encourages nonnutritive sucking, which may facilitate the tube-to-oral (bottle/breast) feeding transition.
Initiation and advancement of feedings
Variation between NICUs and, sometimes, between neonatologists at a single unit is marked regarding when feedings are commonly initiated. Current evidence indicates that trophic or minimal enteral feedings are safe and well tolerated. In this technique, small volumes are fed to infants for a few days without significant increments.
Many neonatologists start feedings within the first week of life and advance the feedings at a rate dependent on gestational age, degree of illness, and other clinical factors. Although some retrospective studies suggest that a rapid increase in feedings may predispose infants to necrotizing enterocolitis, prospective studies have not confirmed this. In general, most neonatologists advance feedings over a period ranging from 5-15 days in ELBW infants and over 4-10 days in neonates weighing 1000-1500g.
OG feedings
Conventionally, infants receive intermittent bolus gavage feedings over 10-20 minutes (by gravity) every 2-3 hours. Feedings may also be administered continuously using an infusion pump. Currently, no evidence strongly indicates that one method of feeding is superior to the other.
Transpyloric feedings
These feedings were initially believed to reduce the risk of gastroesophageal reflux. However, studies have shown a high rate of complications using the transpyloric route, with no additional benefits. Hence, it is not often used unless feeding intolerance using NG or OG tubes is marked. [25]
Fortification
Infants on breast milk are commonly fed fortified breast milk, which increases energy and mineral intake. Infants fed fortified breast milk have had documented improvement in short-term growth and bone mineral content; however, evidence of long-term benefit is insufficient. At present, whether breast milk feeding (with or without fortification) improves long-term neurodevelopment compared with preterm formula is controversial.
Supplementation
Supplementation with long-chain polyunsaturated fatty acids (LCPUFAs), such as docosahexaenoic acid (DHA) and arachidonic acid (ARA), has been recommended for preterm infants on physiologic grounds. Randomized, controlled trials have demonstrated better growth and development in preterm infants fed DHA- or ARA-supplemented formulas. [26, 27] Supplementation of preterm formula with glucose polymers or medium-chain triglycerides improves caloric density, with a slight (< 10%) increase in osmolality at the usual supplemental amounts. [28]
Probiotics
Randomized, controlled trials indicate that supplementation with probiotics (mainly Lactobacillus acidophilus and Bifidobacterium infantis) may reduce the risk of necrotizing enterocolitis and other poor outcomes in preterm infants. [29, 30] Further research is required to determine the agent, dose, and duration, as well as the infants who would benefit most from probiotics.
Feedings at discharge
At discharge, premature infants are usually fed either breast milk or formula (22cal/oz or 20cal/oz). Some evidence suggests that 22cal/oz formula may lead to slightly better nutritional outcomes, probably because of its higher energy, calcium, and phosphate content.
-
An ill preterm infant, such as this patient, requires fluid, electrolyte, and nutrition management in a neonatal intensive care unit.
-
Radiograph depicts necrotizing enterocolitis in a preterm infant. Note the extensive pneumatosis intestinalis and the portal venous air. This situation often requires long-term administration of total parenteral nutrition.
-
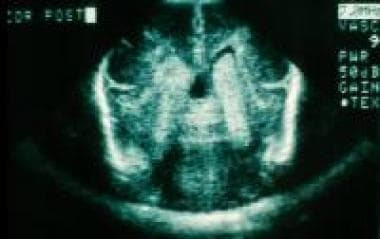
A very low birth weight neonate with a bilateral, grade III intraventricular hemorrhage.
Tables
What would you like to print?
- Overview
- Prevalence and Loss of Body Water
- Assessing Fluid and Electrolyte Status
- Fluid and Electrolyte Management
- Fluids and Electrolytes in Common Neonatal Conditions
- Common Electrolyte Problems
- Oliguria
- Nutritional Requirements
- Total Parenteral Nutrition
- Enteral Nutrition
- Enteral Feeding Methods
- Show All
- Media Gallery
- References