Background
The mitral valve is the inlet valve to the left ventricle (LV). The normal mitral valve is a complex apparatus composed of an annulus and two leaflets that are attached by chordae tendineae to two papillary muscles. The papillary muscles arise from the walls of the LV and secure the chordae and mitral leaflets, preventing prolapse of the valve during ventricular systole.
Proper function of the mitral valve requires an intact mitral valve apparatus and satisfactory LV function. Mitral stenosis (MS) results from any pathologic process that narrows the effective mitral valve orifice at the supravalvular, valvular, or subvalvular levels. MS can be congenital or acquired.
Congenital MS is a rare entity which takes several forms. These forms include hypoplasia of the mitral valve annulus, mitral valve commissural fusion, double orifice mitral valve, shortened or thickened chordae tendineae, and parachute mitral valve in which all chordae attach to a single papillary muscle. [1] The most common associated malformations are coarctation of the aorta, aortic valve stenosis, and subvalvular aortic stenosis. The association of multiple levels of left-sided inflow and outflow tract obstruction is termed the Shone complex. [2]
Severe hypoplasia, or atresia, of the mitral valve results in a hypoplastic LV cavity size that is not capable of sustaining the systemic cardiac output. This situation is considered part of the spectrum of the hypoplastic left heart syndrome and is not considered further in this article. This article deals with MS that, although occasionally severe, allows enough blood flow into the LV to sustain the systemic cardiac output.
Pathophysiology
Mitral stenosis (MS) obstructs blood flow into the left ventricle (LV), elevating left atrial pressure in proportion to severity of the stenosis. This, in turn, restricts pulmonary venous return to the left atrium, elevating pulmonary vascular and, consequently, right heart pressures. Elevated hydrostatic pressure in the pulmonary capillaries forces fluid into the alveoli and interstitial space, producing pulmonary congestion. Congested bronchial veins may encroach on small bronchioles, with subsequent increase in airway resistance.
As a compensatory mechanism, pulmonary vasoconstriction occurs. The right ventricle (RV) pressure increases, resulting in RV hypertrophy. Elevated pulmonary pressure can progress to fixed pulmonary arterial hypertension from medial hypertrophy and intimal thickening of the pulmonary arterioles. The RV eventually fails, and pulmonary blood flow decreases, decreasing systemic blood flow. RV failure results in systemic venous congestion with development of hepatomegaly, ascites, and pedal edema. If the reduction in cardiac output is critical, end organ failure with renal and/or hepatic insufficiency, shock, and metabolic acidosis can occur.
Hemodynamic changes in severe congenital MS are illustrated in the image below.
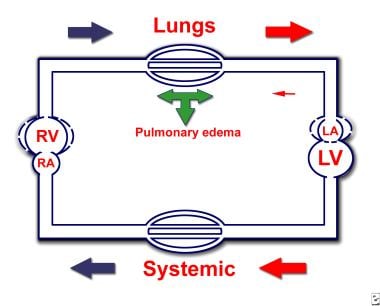 Congenital Mitral Stenosis. Hemodynamic changes in severe congenital mitral valve stenosis (MS). MS causes an obstruction (in diastole) to blood flow from the left atrium (LA) to the left ventricle (LV). Increased LA pressures are transmitted retrograde to pulmonary veins and pulmonary capillaries, resulting in capillary leak with subsequent development of pulmonary edema. To overcome pulmonary edema, the arterioles constrict, increasing pulmonary pressures. With time, capillaries develop intimal thickening, causing fixed (permanent) pulmonary hypertension. The right ventricle (RV) hypertrophies to generate enough pressure to overcome the increased afterload. Eventually, the RV fails, which manifests as hepatomegaly and/or ascites, edema of the extremities, and cardiomegaly on radiography.
Congenital Mitral Stenosis. Hemodynamic changes in severe congenital mitral valve stenosis (MS). MS causes an obstruction (in diastole) to blood flow from the left atrium (LA) to the left ventricle (LV). Increased LA pressures are transmitted retrograde to pulmonary veins and pulmonary capillaries, resulting in capillary leak with subsequent development of pulmonary edema. To overcome pulmonary edema, the arterioles constrict, increasing pulmonary pressures. With time, capillaries develop intimal thickening, causing fixed (permanent) pulmonary hypertension. The right ventricle (RV) hypertrophies to generate enough pressure to overcome the increased afterload. Eventually, the RV fails, which manifests as hepatomegaly and/or ascites, edema of the extremities, and cardiomegaly on radiography.
Etiology
Atrioventricular (AV) valve morphogenesis is a complex process comprised of cell migration, proliferation, apoptosis, and remodeling, all geared towards forming an annulus, AV valve leaflets, and a subvalvar support apparatus comprised of tendinous cords and papillary muscles.
As we know it today, the mitral and tricuspid valves develop at 21 days gestation in the human embryo when endocardial cells migrate into the developing cushion. This is known as the endothelial to mesenchymal transformation (EMT). As these mesenchymal cells proliferate, the endocardial cushions expand and valve primordia appear. The AV canal is then divided into left and right AV valve orifices. The cushions also become populated by epicardial cells as well as cells derived from the second heart field. [3] Individual valve leaflets can be observed as early as week 5 and 6 of fetal development. These valve primordia continue to remodel well into postnatal life, where they become a laminar structure of elastin, collagen, and proteoglycans.
Several genes, including transforming growth factor (TGF)-β, Sox9, and erbB3, are known to contribute to these processes through signaling and downstream activation dependent on the nuclear factor of activated T-cells transcription factors. [3] Therefore, absence of any of such genes will result in AV valve malformations. That genes are responsible for valvar malformation is clear in the fact that the prevalence of MS in offspring of family members with left ventricular outflow tract obstruction is increased, especially if the mother is affected.
In addition, formation of the mitral valve requires proper division of the AV canal as well as commitment of the aortic valve to the left ventricle, which results in fibrous continuity between the anterior (aortic) mitral valve leaflet and the non-coronary and left coronary leaflets of the aortic valve. [4]
Epidemiology
United States data
Congenital mitral stenosis (MS) is rare, occurring in 0.5% of patients with congenital heart disease (CHD).
Race-, sex-, and age-related demographics
No racial or sex predilection is known in congenital MS.
Congenital MS is usually detected in infancy if MS and/or associated heart lesions are severe enough to produce physical findings or to provoke overt symptoms.
Prognosis
Untreated newborns with severe mitral stenosis (MS) have a grim prognosis. Surgical intervention is ideally avoided for as long as possible. Mechanical mitral valve replacement in a small infant or child is a high-risk procedure and carries a guarded prognosis.
Because MS can be associated with other cardiac lesions such as atrial septal defects, ventricular septal defects, left ventricular outflow tract obstruction, and coarctation of the aorta, operative results and long-term outcomes are widely variable and highly dependent on the abnormalities that are present.
Mitral valve replacement entails a less than 5% mortality risk in young, healthy patients without other significant cardiac abnormalities.
Morbidity/mortality
In the fetus, mitral valve obstruction does not interfere with normal growth and development, even if the mitral valve is atretic. This is because the amount of pulmonary venous return to the left atrium is small and the fetal bronchial collateral circulation is adequate to relieve the obstructive effects. In this case, the RV supplies all of the systemic blood flow via the ductus arteriosus, and the patient presents with hypoplastic left heart syndrome.
Less severe forms of MS permit normal fetal circulatory pathways to continue with normal development of the LV and ascending aorta. After birth, if congenital MS is left untreated, morbidity and mortality are high, with mean survival estimated at 3 years. Associated cardiac lesions such as coarctation of the aorta and aortic valve stenosis such as in the Shone complex increase morbidity and mortality.
Complications
If MS is left untreated, the following complications may arise:
-
Pulmonary edema
-
Right heart failure with progression to congestive heart failure
-
Renal insufficiency (due to congestive heart failure)
-
Progression to pulmonary hypertension
-
Atrial arrhythmias: Atrial arrhythmias such as fibrillation or flutter occur more frequently in patients with chronic left atrial enlargement. Initiation and perpetuation of these arrhythmias has been attributed to a vertical line of conduction delay that runs between the pulmonary veins.
-
Thrombus formation in the dilated left atrium (due to stasis of blood)
-
Embolization of a left atrial thrombus, stroke
-
Dysphagia from compression of the esophagus by an enlarged left atrium
Complications of medical treatment include the following:
-
Diuretics may provoke dehydration (decreased preload) with subsequent compromise in cardiac output that may precipitate prerenal renal failure.
-
Warfarin may cause bleeding, such as intracranial hemorrhage and gastrointestinal (GI) bleeding.
Complications of surgery include the following:
-
Patients with associated congenital cardiac anomalies have a higher risk of early death after mitral valve surgery.
-
The risks of mitral valve replacement include those associated with anticoagulation, valve thrombosis, valve dehiscence, infective endocarditis, valve malfunction, and embolic events.
-
Iatrogenic mitral valve insufficiency may occur as a result of surgery (or balloon dilatation, when performed) of the stenotic mitral valve. This can be corrected immediately as repairs are assessed by intraoperative transesophageal echocardiography.
-
Mitral commissurotomy may cause significant postoperative mitral regurgitation, which may necessitate subsequent mitral valve replacement.
-
Patients with associated left ventricular outflow tract obstruction who require subaortic membrane resection may develop complete atrioventricular block and require a permanent pacemaker.
Complications of percutaneous balloon valvuloplasty include the following:
-
Safety depends on the mitral valve morphology and on the operator's experience. Very few forms of congenital MS are amenable to balloon valvotomy. Percutaneous balloon valvotomy should not be performed in patients with pre-existing moderate-to-severe mitral valve regurgitation.
-
The most frequent complication after percutaneous balloon valvuloplasty is mitral regurgitation.
Patient Education
Counsel the patient and families regarding the appearance and worsening of symptoms of mitral stenosis.
Prior to any invasive or surgical procedure, advise the patient regarding subacute bacterial endocarditis (SBE) prophylaxis.
Monitor prothrombin time (PT) and international normalized ratio (INR) if the patient is on anticoagulation medication.
Advise pregnant mothers to avoid taking warfarin due to teratogenicity, avoid strenuous activity and excessive salt intake, and have their blood pressure frequently monitored.
For patient education resources, see the Heart Health Center, as well as Mitral Valve Prolapse.
-
Congenital Mitral Stenosis. Hemodynamic changes in severe congenital mitral valve stenosis (MS). MS causes an obstruction (in diastole) to blood flow from the left atrium (LA) to the left ventricle (LV). Increased LA pressures are transmitted retrograde to pulmonary veins and pulmonary capillaries, resulting in capillary leak with subsequent development of pulmonary edema. To overcome pulmonary edema, the arterioles constrict, increasing pulmonary pressures. With time, capillaries develop intimal thickening, causing fixed (permanent) pulmonary hypertension. The right ventricle (RV) hypertrophies to generate enough pressure to overcome the increased afterload. Eventually, the RV fails, which manifests as hepatomegaly and/or ascites, edema of the extremities, and cardiomegaly on radiography.
-
Congenital Mitral Stenosis. Two-dimensional echocardiograph, parasternal long axis view of a 5-month-old boy with congenital mitral valve stenosis. A small mitral valve annulus (star) is appreciated when compared with the normal-sized tricuspid valve annulus. Mitral valve stenosis has caused left atrial (LA) enlargement. AoV = Aorta; LA = Left atrium; LV = Left ventricle; RA = Right atrium; RV = Right ventricle.
-
Congenital Mitral Stenosis. Two-dimensional echocardiograph, parasternal long axis view of a patient who required mitral valve replacement with a St. Jude's prosthetic mitral valve (star). He developed a stroke one month after mitral valve replacement despite anticoagulation with warfarin and required re-replacement of the prosthetic mitral valve. He will eventually outgrow this new prosthetic mitral valve and require subsequent mitral valve replacements with a larger mitral valve prosthesis. AoV = Aorta; LA = Left atrium; LV = Left ventricle; RA = Right atrium; RV = Right ventricle.







