Background
Left bundle branch (LBB) block (LBBB) occurs when transmission of the cardiac electrical impulse is delayed or fails to be conducted along the rapidly conducting fibers of the main LBB or in both left anterior and posterior fascicles. [1] Thus, the left ventricle slowly depolarizes by means of cell-to-cell conduction that spreads from the right ventricle to the left ventricle. This results in the characteristic electrocardiographic (ECG) pattern shown in the image below.
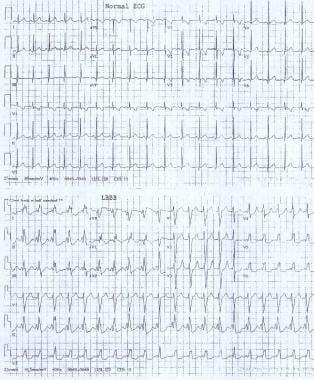 These electrocardiograms show a normal sinus rhythm and a sinus rhythm with a left bundle branch block.
These electrocardiograms show a normal sinus rhythm and a sinus rhythm with a left bundle branch block.
LBBB in children is associated with cardiovascular disease, surgery, or ablation within the left ventricle and is not observed in the general population. LBBB may occur in as many as 20% of individuals after aortic valve replacement.
See Are You Missing Subtle MI Clues on ECGs? Test Your Skills, a Critical Images slideshow, to help identify a variety of electrocardiographic abnormalities.
Pathophysiology
Knowledge of the anatomy and electrophysiology of the cardiac conduction system from the atrioventricular (AV) junction to the distal Purkinje fibers is essential to understanding the pathophysiology of left bundle branch (LBB) block (LBBB).
Embryology
During cardiogenesis, the differentiation into working muscle cells and the conduction system remains a matter of discussion. Hypotheses have been developed that, singularly or in combination, could explain this complex subject. The components of the cardiac pacemaker and conduction system are not uniform with respect to the function, morphology, and molecular phenotype. Several models and theories have been formed. The four-ring theory describes four conduction system rings within the cardiac tube that differentiate into components of the pacemaker and conduction system. The recruitment model assumes some cardiomyocytes are committed early on to the conduction system. The specification model postulates that the primary myocardial cells express either conduction system or working myocyte genes. [2]
Anatomy
The specialized conduction system of the heart is composed of cells that conduct electrical impulses faster than the surrounding myocardium. The conduction system can be divided into distinct anatomic segments, and each segment is described in sequence beginning at the AV junction and ending with the Purkinje fibers.
The AV junction has traditionally been divided into three regions as follows: transitional cell zone, AV node, and penetrating portion of the AV bundle (His bundle, common bundle).
The transitional cell zone is where the right atrium merges with the compact AV node by means of discrete atrial pathways termed the slow and fast pathways. In the past, the slow and fast pathways were believed to be parallel. However, data from radiofrequency ablations of the AV nodal and AV reentrant tachycardias have demonstrated that the slow pathway is more closely associated with anterior-superior aspect of the os of the coronary sinus. Furthermore, the data show the fast pathway is located slightly superior and posterior to the AV node.
The next segment is the AV node, which lies anterior and superior to the ostium of the coronary sinus, directly above the insertion of the septal leaflet of the tricuspid valve. This area is located at the apex of the triangle of Koch, which is formed by the tricuspid annulus, the tendon of Todaro, and the ostium of the coronary sinus. Blood supply to the AV node is derived from the AV nodal artery, which is a branch of the right coronary artery in 85%-90% of individuals and a branch of the left circumflex coronary artery in 10%-15% of individuals.
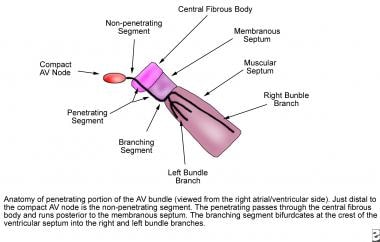
At the apex of the triangle of Koch, the compact AV node becomes the penetrating bundle of His. It penetrates the central fibrous body at the attachment of the tendon of Todaro, runs between the membranous septum and the muscular septum, and bifurcates at the crest of the muscular ventricular septum. The His bundle is divided into three anatomic segments. The proximal, or nonpenetrating, segment lies distal to the AV node and proximal to the central fibrous body. The middle, or penetrating, segment penetrates the central fibrous body and runs posterior to the membranous septum. The distal, or branching, segment bifurcates at the crest of the muscular septum into the right and left bundle branches (see the image below).
The right bundle branch, a direct continuation of the penetrating bundle, originates distal to the attachment of the septal leaflet of the tricuspid valve with the membranous septum and surfaces on the right ventricular septum just below the papillary muscle of the conus. It is unbranched and proceeds toward the apex of the right ventricle along the posterior margin of the septal band, courses through the moderator band to the base of the anterior papillary muscle, and proceeds to the right ventricular free wall.
The LBB originates at the crest of the muscular ventricular septum just distal to the membranous septum. It arises in a fanlike fashion and descends inferiorly along the left ventricular septal surface beneath the noncoronary cusp of the aortic valve. The LBB usually branches into three major fascicles. The anterior fascicle is directed to the base of the anterolateral papillary muscle, the posterior fascicle is directed to the base of the posteromedial papillary muscle, and, in 60% of hearts, a central fascicle proceeds to the midseptal region. When no central fascicle is present, as in 40% of hearts, the midseptal region is supplied by radiations from the anterior fascicle or the anterior and posterior fascicles.
At the terminal aspect of each bundle branch, Purkinje fibers are interlaced on the endocardial surface of both ventricles and tend to be concentrated at the tips of the papillary muscles.
Electrophysiology of cardiac conduction
The heart is a two-step mechanical pump coordinated by precisely timed electrical impulses. For the pump to perform optimally, sequential depolarizations of the atria and then the ventricles allow atrial contraction to provide complete diastolic filling of the ventricles (AV synchrony). After the ventricles are filled, rapid activation of the ventricular myocardium permits a synchronized contraction to eject blood most effectively to the great vessels.
Normal cardiac conduction
In normal cardiac conduction, electrical excitation of the heart proceeds in a sequential manner from the atria to the ventricles and is demonstrated on the surface electrocardiogram (ECG) (see the image below).
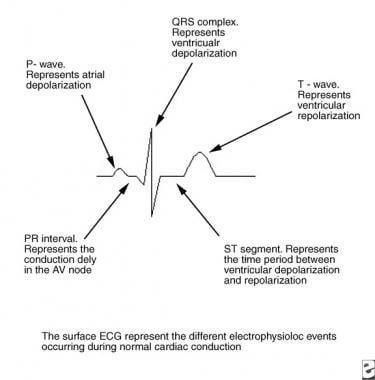 This image depicts the electrophysiologic events in normal cardiac conduction. AV = atrioventricular; ECG = electrocardiogram.
This image depicts the electrophysiologic events in normal cardiac conduction. AV = atrioventricular; ECG = electrocardiogram.
The electrical impulse generated in the sinus node proceeds through the atria (reflected by the P wave on the ECG) to reach the AV node. As the impulse conducts through the AV node, conduction slows, allowing time for atrial contraction to occur before the ventricle is activated (PR segment). After the impulse passes through the compact AV node, it is rapidly conducted through the crux of the heart to the ventricles by means of the bundle of His (penetrating bundle) to the branching bundle, the bundle branches, the distal Purkinje fibers, and, finally, the ventricular myocardial cells (narrow QRS complex).
The anatomy of the LBB is variable. [3] The origin of the LBB is broad in some and narrow in others (ranging from < 1 mm to 14 mm) and is significantly influenced by the anatomic relationship of the His bundle to the ventricular septum. The subdivision of the LBB is also greatly variable. Some studies suggest a trifascicular division of the LBB into an anterior, posterior and septal branch. For clinicians and electrophysiologists, the concept of bifascicular (anterior and posterior fascicle) LBB has served well. [4] In a normal heart, the electrical impulse arriving from the His bundle results in a parallel and independent activation of both ventricles, whereas the ventricular septum is activated in a left-to -ight direction. The rich Purkinjie network on each side transmits the electrical impulse to the myocardial cells. [5]
Types of left bundle branch block
Complete LBBB occurs when the electrical impulse is delayed or interrupted in either the main LBB or in both the anterior and posterior fascicles. Conduction down the right bundle branch normally proceeds, and the right ventricle depolarizes in the normal fashion. In complete LBBB, conduction from the right ventricle passes first to the interventricular septum, then to the anterior and posterior portions of the left ventricle, and finally to the left lateral free wall. Delayed left ventricular depolarization accounts for the ECG findings in left bundle branch block (see the images below).
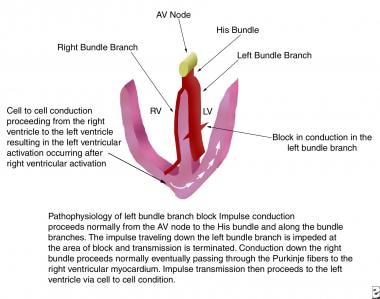 This image depicts the pathophysiology of left bundle branch block. AV = atrioventricular; LV = left ventricle; RV = right ventricle.
This image depicts the pathophysiology of left bundle branch block. AV = atrioventricular; LV = left ventricle; RV = right ventricle.
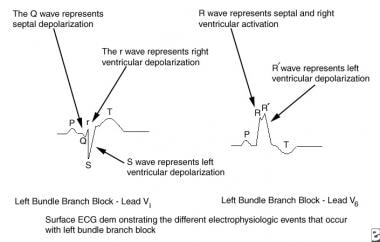 This image depicts the electrophysiologic events of left bundle branch block. ECG = electrocardiogram.
This image depicts the electrophysiologic events of left bundle branch block. ECG = electrocardiogram.
Incomplete LBBB occurs in two forms, each called hemiblock. In left anterior hemiblock (LAH), transmission of the electrical impulse proceeds normally along the main LBB and the posterior fascicle, but it is blocked or delayed in the anterior fascicle. This blockage results in delayed activation of the anterior portion of the left ventricle. In LAH, the duration of the QRS complex may be of normal or only slightly prolonged duration because of normal rapid conduction down the right and left main bundle and the left posterior fascicle. In addition, the QRS complex is directed superiorly in the frontal plane. This is called left axis deviation, although the term superior axis deviation most accurately describes the finding. Furthermore, QRS axis is normally to the left; therefore, the term left axis deviation makes little semantic sense.
With left posterior hemiblock, transmission of the electrical impulse proceeds normally along the main LBB and the anterior fascicle, but it is blocked in the posterior fascicle. This blockage results in delayed activation of the posterior left ventricle. The QRS complex is again of normal or only slightly prolonged duration and inscribes a rightward axis in the frontal plane. Left posterior hemiblock is rarely observed in children, and diagnosis is difficult because of the common association of right axis deviation in children with congenital heart disease and right ventricular hypertrophy.
Epidemiology
Etiology
Left bundle branch block
Left bundle branch (LBB) block (LBBB) is not a benign entity; risk factors include the following:
-
LBBB is associated with anatomic malformations and abnormalities of the conduction system (eg, as is observed in Lenegre disease).
-
LBBB has been observed after surgery in the left ventricular outflow tract, septal myectomy, alcohol septal ablation, replacement of the aortic valve, radiofrequency ablation, and transcatheter closure of perimembranous ventricular septal defects.
-
LBBB is also observed in patients with left ventricular hypertrophy, left ventricular noncompaction and neuromuscular disease, progressive conduction system disease, myocarditis, [6] cardiomyopathy, [7] hemochromatosis, sclerodegenerative diseases, myocardial infarction, aortic valve endocarditis, rheumatic fever with aortic valve involvement, perinatal exposure to HIV type 1, [8] Wolff-Parkinson-White Syndrome (when the abnormal conduction pathway enters the right ventricle), and acute pulmonary embolism. [9] (See also Supraventricular Tachycardia, Wolff-Parkinson-White Syndrome.)
-
The LBBB pattern, or rather, left ventricular conduction delay, also occurs in patients with Wolff-Parkinson-White Syndrome in whom the abnormal conduction pathway enters the right ventricle.
-
Some patients may demonstrate a LBBB pattern during supraventricular tachycardia (ie, rate-dependent bundle branch block).
-
A case report in an infant with LBBB and left ventricular noncompaction and four gene mutations (HCN4(G811E), SCN5A(L1988R), DMD(S2384Y), EMD(R203H)) indicates that other (unknown) genetic factors in combination with mutations in HCN4(G811E) (which result in a G811E substitution in hyperpolarization-activated cyclic nucleotide-gated channel 4, the main subunit of the cardiac pacemaker channel) may cause these cardiac conditions. [10]
Left anterior hemiblock
Risk factors for left anterior hemiblock (LAH) include the following:
-
LAH has been associated with coronary artery disease, left ventricular hypertrophy, cardiomyopathy, tetralogy of Fallot repair, ventricular septal defect repair, septal myectomy, subvalvar aortic resection, and an anomalous origin of the left coronary artery from the pulmonary artery.
-
LAH may be present in patients with autosomal dominant bundle branch disease and has been associated with lentiginosis.
-
Among congenital heart defects, LAH is characteristic of endocardial cushion defects, such as ostium primum atrial septal defect complex and complete AV canal, an abnormality frequently seen in patients with Down syndrome. LAH occurs in endocardial cushion defects because of congenital absence or hypoplasia of the left anterior division. LAH has also been described in Noonan syndrome.
-
Patients with endocardial cushion defects (ostium primum atrial septal defect, complete AV canal), tricuspid atresia, double-outlet right ventricle, and certain forms of a functional single ventricle have LAH that may reflect absent, hypoplastic, or abnormally coursing left bundle fascicles rather than an anterior hemiblock per se.
Risk factors for left posterior hemiblock
Left posterior hemiblock is observed after congenital heart surgery and in patients with congenital aortic stenosis, endocarditis, or diphtheritic myocarditis.
Prognosis
Morbidity/mortality
Progression to complete heart block or sudden death is rare, and the prognosis depends on associated systemic or cardiovascular disease more than the left bundle branch block (LBBB) itself. Biventricular pacing may improve clinically significant and progressive morbidity associated with concomitant congestive heart failure.
Patients with LBBB, left axis deviation, and first-degree heart block or LBBB associated with near-syncope or syncope require close follow-up and/or electrophysiologic study. In adults, LBBB can disturb coronary perfusion of the left anterior descending coronary artery by shortening the duration of diastolic flow.
As noted, if LBBB progresses to complete heart block, the patient may have syncope or suddenly die.
-
These electrocardiograms show a normal sinus rhythm and a sinus rhythm with a left bundle branch block.
-
This image depicts the anatomy of the penetrating portion of the atrioventricular (AV) bundle.
-
This image depicts the electrophysiologic events in normal cardiac conduction. AV = atrioventricular; ECG = electrocardiogram.
-
This image depicts the pathophysiology of left bundle branch block. AV = atrioventricular; LV = left ventricle; RV = right ventricle.
-
This image depicts the electrophysiologic events of left bundle branch block. ECG = electrocardiogram.