Background
Subvalvar aortic stenosis (SAS), also called subaortic stenosis, is a fixed form of anatomic obstruction to egress of blood across the left ventricular outflow tract (LVOT). [1] Although classified as a congenital heart defect, its rarity at birth and during infancy, its progressive course, and its high rate of postoperative recurrence suggest that it may be an acquired condition. Images of SAS appear below. [2] (See Etiology.)
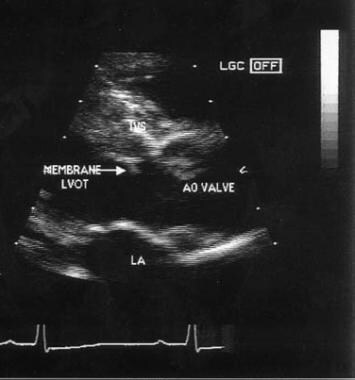 Echocardiogram of membranous subaortic stenosis. AO = aortic; LA = left atrium; LVOT = left ventricular outflow tract.
Echocardiogram of membranous subaortic stenosis. AO = aortic; LA = left atrium; LVOT = left ventricular outflow tract.
SAS has a spectrum of anatomic variants and usually has a variable, progressive course. Associated congenital heart defects are found in 25-50% of patients with SAS; the most common defects include ventricular septal defect (VSD), patent ductus arteriosus, coarctation of aorta, bicuspid aortic valve, abnormal left ventricular (LV) papillary muscle, atrioventricular septal defect, Shone complex, interrupted aortic arch, and persistent superior left vena cava. (See Diagnosis.)
In most patients, SAS is detected in the course of follow-up care for associated congenital heart defects following previous cardiac surgery or during evaluation of a heart murmur. Many patients are asymptomatic. Among symptomatic patients, exertional dyspnea is the most common manifestation. (See Presentation.)
Two-dimensional (2D) echocardiography with color Doppler imaging is the current modality of choice to establish the diagnosis of SAS (see Workup). The surgery of choice for discrete fibromuscular SAS is complete resection with myotomy, with or without myomectomy through an aortotomy. Children, adolescents, and young adults with clinically significant aortic regurgitation, which is usually a consequence of the subaortic stenosis, may require aortic valve repair or replacement. (See Pathophysiology and Treatment.)
Go to Aortic Stenosis for more complete information on this topic.
Anatomy
The boundaries of the left ventricular outflow tract (LVOT) are formed posterolaterally by the anterior leaflet of the mitral valve and intravalvar fibrosa and anteromedially by the muscular and membranous portions of the interventricular septum.
Anatomic variants in SAS
Fixed lesions of the LVOT that cause subvalvar aortic stenosis (SAS) have a spectrum of morphologies. The 4 basic anatomic variants are as follows: (1) a thin discrete membrane consisting of endocardial fold and fibrous tissue, (2) a fibromuscular ridge consisting of a thickened membrane with a muscular base at the crest of the interventricular septum, (3) a diffuse, fibromuscular, tunnel-like narrowing of the LVOT, [3, 4] and (4) accessory or anomalous mitral valve tissue.
Types 1 and 2 account for 70-80% of all cases of SAS. Located 0.5-1.5 cm beneath the aortic valve, types 1 and 2 involve a variable extent of the LVOT. Following previous surgical ventricular septal defect patch closure, a steep (>130°) aortoventricular septal angle, increased mitral-aortic separation, and an exaggerated aortic override are present in children who later develop SAS.
LVOT obstructions secondary to accessory tissue, an anomalous basal attachment of the anterior mitral leaflet, and an anomalous chordal attachment of mitral valve occur, but they are uncommon. [5]
Pathophysiology
Abnormalities of the aortic and mitral valves frequently develop during the natural course of subvalvar aortic stenosis (SAS) because of tethering by encroaching fibroelastic tissue of the membrane and fibromuscular ridge.
Clinically significant obstruction to ejection due to SAS results in concentric left ventricular (LV) hypertrophy, often with an excessive septal bulge. This effect leads to a cycle of further obstruction and localized fibromuscular growth.
SAS has variable and unpredictable rates of progression in children, whereas the rate of progression in adults is slow.
Heart failure occurs only occasionally in pediatric patients. When present early in life, heart failure usually results from associated congenital heart defects. Cardiac output usually is well maintained, and systolic function is well preserved in children with isolated SAS, until severe obstruction develops.
Aortic regurgitation
Thickening of the aortic valve and mild, asymmetrical, poststenotic dilatation of the ascending aorta may be, in part, due to repetitive trauma and vibrations from the high-velocity jet of blood flow through the site of stenosis. Aortic regurgitation, which is usually progressive, develops in nearly 65% of patients in the course of SAS, and it usually persists even after the SAS is removed.
The detection of aortic regurgitation is a function of timely follow-up care. Although the aortic regurgitation is usually mild, its incidence and severity increases with an increasing LV outflow tract (LVOT) pressure gradient. This finding may reflect progressive damage to the aortic valve by the high-velocity jet of blood that SAS produces.
Aortic regurgitation adds volume overload to an already pressure-overloaded LV. The resultant decreased aortic diastolic pressure leads to diminished coronary perfusion and, in combination with increased left ventricular oxygen demand from pressure and volume overload, predisposes the left ventricular myocardium to ischemic injury. In some patients, progressive aortic regurgitation requires aortic valve repair or replacement at the time of surgical intervention for SAS. SAS may recur even after surgical resection appears to be complete and is usually a consequence of abnormal left ventricular outflow tract geometry.
Etiology
The etiology of subvalvar aortic stenosis (SAS) still is not fully understood. Fixed SAS may be the postnatal expression of a latent congenital lesion brought out by many mechanisms, such as genetic predisposition, certain anatomic characteristics of the left ventricular outflow tract (LVOT), hemodynamic abnormalities associated with other cardiac lesions, or surgical interventions that result in chronic flow disturbance in the outflow tract. [6] No genetic inheritance is known for SAS, and few familial incidences are reported. [7]
SAS may have a principally morphogenetic basis, whereby anatomical variants lead to abnormal cell proliferation and morphologic changes due to abnormal flow patterns. [3, 8, 9] Anatomic characteristics that may promote a chronic flow disturbance in the LVOT include a long, narrow LVOT; a steep (>130°) aortoventricular septal angle; increased mitral-aortic separation; and exaggerated aortic override. A focal myocardial abnormality similar to that found in hypertrophic cardiomyopathy may also play a role. The effect of abnormal flow patterns on a genetically vulnerable myocardium may account for the development of SAS in some cases.
Such LVOT morphology may inherently increase fluid shear stress on the interventricular septum and induce an abnormal endothelial and muscle-proliferative response in the outflow tract, with eventual formation of a fibromuscular ridge.
A possible hemodynamic basis of SAS is that alternation in left-sided flow before and after repair of associated congenital heart defects likely causes turbulence in the LVOT and adds to fluid shear stress on the interventricular septum. Therefore, morphologic abnormalities may result in flow disturbance that may be instrumental in the formation of SAS.
A possible genetic etiology in humans has not been identified. However, the Newfoundland dog has been shown to experience an increased incidence of subaortic membranes, which may in part be secondary to inbreeding and is consistent with an autosomal inheritance. [10]
Epidemiology
The approximate incidence of congenital heart defects is approximately 8 per 1000 live births. Subvalvar aortic stenosis (SAS) accounts for approximately 1% of all congenital heart defects (8 in 10,000 births) and for 15-20% of all fixed left ventricular outflow tract (LVOT) obstructive lesions. [11]
The male-to-female ratio of SAS is 2:1 to 3:1. [12] Distinctions in the natural history and postoperative course of SAS between male and female patients have not been clearly defined. However, more male than female patients require repeat surgery. Isolated SAS is rarely seen at birth or during infancy. SAS may develop in some patients after they undergo repair of associated congenital heart defects (eg, ventricular septal defect), usually by age 2 years. Exceptions include patients with Shone complex and interrupted aortic arch, who can have SAS in early infancy.
Prognosis
Although natural history studies have not delineated the annual mortality rate, 2-10% of sudden deaths are reported in untreated individuals with severe left ventricular outflow tract (LVOT) obstruction, including subvalvar aortic stenosis (SAS), valvar aortic stenosis, and supravalvar aortic stenosis.
Postoperative survival rates are at least 85-95% at 15 years. [13] Late mortality is mostly related to residual LVOT obstruction and repeat operation.
The high postoperative recurrence rate (10-50% on >10-y follow-up) has been associated with a high preoperative LVOT pressure gradient (>50 mm Hg), tunnel-like lesions, incomplete removal of discrete SAS, and age younger than 10 years at surgery. The Ross-Konno procedure seems to reduce the postoperative recurrence rate for tunnel-like SAS. [14]
Overall surgical mortality for SAS is now less than 1% in most centers. [15, 16, 17, 18]
Complications
Preoperative complications include the following:
-
Progressive aortic valve damage
-
Ventricular dysfunction
-
Infective endocarditis
-
Sudden death
Sudden cardiac death, unlike in hypertrophic cardiomyopathy, is reported in only a small percentage of patients with SAS. Sudden cardiac death is usually not the first clinical manifestation of the disease. It almost always occurs in previously symptomatic patients, typically those who have an echo Doppler LVOT pressure gradient of more than 50 mm Hg.
Postoperative complications include the following:
-
Damage to the aortic or mitral valve or both (< 2% of patients)
-
Variable degree of heart block (< 2-5% of patients) or isolated left anterior hemiblock or complete left bundle branch block
-
Iatrogenic ventricular septal defect (VSD; < 2% of patients)
-
Infective endocarditis
Bacterial endocarditis is uncommon and most likely to occur in patients with a damaged aortic valve. Bacterial endocarditis can also result in hemodynamically significant aortic regurgitation and congestive heart failure in patients with SAS.
-
Echocardiogram of membranous subaortic stenosis. AO = aortic; LA = left atrium; LVOT = left ventricular outflow tract.
-
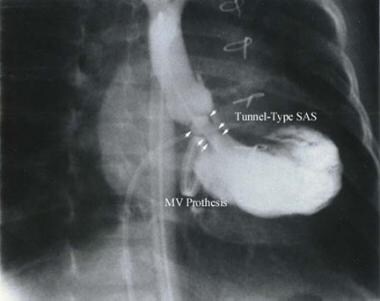
Tunnel-type of subaortic stenosis (subvalvular aortic stenosis [SAS]). MV = mitral valve.