Practice Essentials
Yellow fever (YF) is a mosquito-borne infection that is caused by the YF virus and is endemic to Africa and South America. It is characterized by variable symptoms ranging from a minimal flulike illness to one that may be complicated by a toxic phase characterized by hemorrhage, hepatic failure, proteinuria, renal failure, and death.
Signs and symptoms
YF is an illness characterized by acute onset of fever followed by jaundice within 2 weeks of the onset of first symptoms plus one of the following:
-
Bleeding from the nose, gums, gastrointestinal (GI) tract, or skin
-
Death within 3 weeks of illness onset
See Presentation for more detail.
Diagnosis
The diagnosis of YF is made on the basis of any one of the following:
-
Isolation of YF virus
-
Isolation of YF virus–specific immunoglobulin M (IgM)
-
Fourfold or greater rise in serum immunoglobulin G (IgG)
-
Positive findings on postmortem liver histopathology
-
Detection of YF antigen in tissues with immunohistochemistry
-
Detection of YF viral genomic sequences with polymerase chain reaction (PCR) evaluation
See Workup for more detail.
Management
No specific treatment for YF is noted. Supportive care is the mainstay of treatment. Base management decisions on the presence of dehydration, electrolyte imbalance, organ failure, concurrent infections, secondary infections, hemorrhagic diathesis, and generalized symptoms.
Prevention remains the cornerstone to minimizing the risk of YF. Travelers to endemic areas and local populations should be vaccinated.
See Treatment and Medication for more detail.
Background
Overall, YF reemergence has occurred since 1985, as reflected in the number of cases per year officially reported to the World Health Organization (WHO). Approximately 200,000 cases occur annually; 80-90% of those reported are from sub-Saharan Africa, primarily West Africa. YF is reemerging in South America. In addition, Aedes aegypti has repopulated large swaths of Central America, as well as the Caribbean coastal areas of the United States and most of Florida, allowing the potential reemergence of yellow fever in these areas. [1]
Supportive care is the only treatment. Mortality is 20-50%. Prevention using the live-attenuated 17D vaccine is highly efficacious.
Immediately report all suspected or confirmed cases of YF to local and state health departments, which then report immediately to the Division of Global Migration and Quarantine (1-404-498-1600) or Division of Vector-Borne Infectious Diseases (1-970-221-6400), Centers for Disease Control and Prevention (CDC). If local or state health departments cannot be reached, contact the CDC directly.
Pathophysiology
YF virus, an arbovirus, is the type species for the family Flaviviridae and is a single, positive-stranded, enveloped RNA virus. The envelope consists of a lipid bilayer containing an envelope glycoprotein and a matrix protein. The single RNA is complexed with a capsid protein. Viral strains from South America are closely related to those from West Africa. This observation supports the supposition that YF virus originated in West Africa.
The virus is transferred from the infected female mosquito’s salivary gland by means of saliva introduced into a bite wound during a blood meal. It replicates in local tissues and regional lymph nodes. The virus can then infect a feeding mosquito during the initial 3-4 days of the illness. No human-to-human transmission is known.
After the virus enters the bloodstream, hematogenous spread to the bone marrow, kidney (probable), liver (the main target), myocardium, and spleen ensues, and further replication occurs. Cerebral edema and cerebral petechial hemorrhages result from secondary factors. In the hemorrhagic diathesis that may follow, disseminated intravascular coagulation (DIC) involves decreased synthesis of coagulation factors, altered platelet function, and bleeding from the gastrointestinal (GI) mucosa and abdominal or pleural serosa.
Myocardial fiber injury occurs secondary to direct virus activity, with cloudy swelling and fatty change. Shock and death can result. Multiple organ insult involves the liver, kidneys, brain, and heart. Other consequences include hemorrhage and secondary effects of vasoactive cytokines. In an immune response, viral neutralizing antibodies are present by the end of the first week, and the virus is rapidly cleared. Immune response confers lifelong immunity. The role of immune response in pathogenesis has not yet been established.
Etiology
YF is a mosquito-borne infection that is caused by the YF virus. Mosquitoes that spread the virus include A aegypti (also carries dengue and chikungunya viruses; see the image below), other Aedes species, and Haemagogus species.
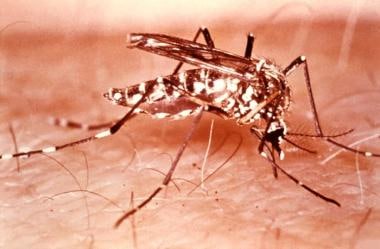 This female Aedes aegypti mosquito is shown here after landing on a human host. The A aegypti mosquito is a known transmitter of both dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when bitten by a female mosquito. Image courtesy of the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
This female Aedes aegypti mosquito is shown here after landing on a human host. The A aegypti mosquito is a known transmitter of both dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when bitten by a female mosquito. Image courtesy of the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).
These mosquitos are diurnal (daytime) feeders, and only females feed on blood. In humans, YF virus is transmitted to the mosquito from an ill human only during the initial 3-4 days of illness. If the female is interrupted while feeding, it may seek another host on which to feed, leading to multiple transmissions during a feeding cycle. The extrinsic incubation period (ie, the interval from the time when the mosquito is infected with YF virus to the time when the mosquito can transmit the virus to another host) is 12-21 days.
YF virus enters the ovum in the female mosquito and survives there during the dry season. Fewer than 1% of female mosquito offspring are infected. Uninfected female mosquitoes bite infected hosts, providing for viral amplification. This is essential for survival of the virus. Approximately 3-10 virions are necessary to infect a mosquito.
Mosquitoes breed in stagnant water, including rainwater in tree holes, human-made water storage vessels, used vehicle tires, and in other collections of water in and about dwellings (eg, broken coconut shells, flower vases, gutters, tin cans, and water drums). Seasonal transmission occurs, with peak transmission usually being the time of peak humidity and rainfall. In Africa, the peak season is from the middle of the rainy season to the early dry season. In South America, it is from January to March. However, transmission is not limited to the peak season.
Transmission patterns
Jungle, urban, and intermediate (savannah) cycles of transmission have been identified. In some publications, the term sylvatic cycle (from Latin silva or sylva “woods, forest”) is used as a synonym for intermediate cycle; in others, it is used as a synonym for jungle cycle.
Jungle cycle
The mosquito transmits the virus to wild nonhuman primates (or possibly an incidental human host), from which it is transmitted to another mosquito. This cycle is confined to rain forests, and human hosts are usually males working in the forests clearing trees. In Africa, Aedes species not including A aegypti are implicated, whereas in South America, Haemagogus species are involved.
Urban cycle
The mosquito transmits the virus to a human host, from whom it is transmitted to another mosquito. In this cycle, which is confined to urban areas, A aegypti, a domestic mosquito that breeds in human-made containers, is the primary vector.
Intermediate (savannah) cycle
The mosquito transmits the virus to wild nonhuman primates and human hosts, and the virus is then transmitted to another mosquito. This cycle, confined to moist savannas and forest savanna ecotones of Africa, is the most common cycle for outbreaks in Africa. Many villages in a given area are affected simultaneously. Mortality appears to be lower with these outbreaks. Semidomestic mosquitoes that live in and outside of villages are the primary vectors.
This cycle may act as a bridge between the jungle and urban areas, leading to spread to urban centers, where an urban epidemic can result if contact is made between domestic mosquitoes and an unvaccinated human population.
Epidemiology
In Africa, YF occurs in areas between 15° north and 10° south of the Equator, a region encompassing 34 countries with a total population of nearly 500 million people. [2] In South America and the Caribbean, enzootic countries include Bolivia, Brazil, British Guyana, Colombia, Ecuador, French Guyana, Panama (south of the Canal Zone), Peru, Surinam, Trinidad, and Venezuela. Human cases were reported in the 1990s in Bolivia, Brazil, Colombia, Ecuador, and Peru.
In Asia, although conditions currently exist for the transmission of YF in Asia (eg, on the Indian subcontinent), no documented transmission has ever occurred there. Evidence suggests that previous infection with another flavivirus (eg, those causing dengue) may confer protection from YF. This has been cited as the reason why YF has not been observed in Asia.
United States statistics
Since 1996, 3 fatal cases of YF have been reported in American travelers to the Amazon. [3, 4, 5] None of the patients were immunized against YF; 2 of them did receive other pretravel vaccinations, including the hepatitis A vaccine, but the third did not receive a medical consultation, YF vaccine, or malaria prophylaxis before traveling. Because patients with YF can present with a mild influenzalike illness, YF may not be recognized and may go unreported.
The outfitter of 1 of the patients stated, “The International medical community suggests yellow fever and malaria prophylaxis for the Amazon region. This is not a requirement to enter Brazil, but merely a suggestion.” The brochure of a travel agent stated, “We do not suggest any inoculations of any kind for this trip.... But to make sure you are worry free, consult with your personal physician.” These cases illustrate that some outfitters, travel agents, and physicians may underestimate health risks of travelers. [5]
In 1996, a 45-year-old man spent 9 days in the jungles of Brazil. [3] He returned to the United States with headache, myalgias, arthralgias, and chills. During an initial visit to an emergency department (ED), he had fever, leukopenia, thrombocytopenia, and mild elevations in hepatic transaminase values. He subsequently developed jaundice and hemorrhagic manifestations and died 10 days after developing symptoms. YF virus was isolated from tissue specimens.
In 1999, a 48-year-old man returned to the United States after a 10-day trip to a forested area of Venezuela. [4] During his trip, he received multiple mosquito bites. On the day of his return, he developed fever, chills, headache, photophobia, myalgias, arthralgias, nausea, vomiting, constipation, upper abdominal discomfort, and weakness. He developed hemorrhagic manifestations and died 9 days after developing symptoms. YF viral antigens were isolated from postmortem liver specimens by using immunohistochemical methods.
In 2002, a 47-year-old man traveled to the Brazilian Amazon to fish. [5] He slept in an air-conditioned boat and wore clothing impregnated with N,N -diethyl-m-toluamide (DEET). He returned to Texas and presented to an ED the same day, complaining of 4 days of crampy abdominal pain, 1 day of fever (102.8°F [39.3°C]), and severe headache. He was treated for presumed rickettsial infection and sent home.
Two days later, this patient was admitted for intractable vomiting. On initial evaluation, he had leukopenia (white blood cell [WBC] count 2.3 × 109/L [2300/µL]), coagulopathy, hepatic failure, and renal failure. He was treated for malaria. Bacterial cultures of blood, urine, and cerebrospinal fluid (CSF) were negative, as were malaria smears. On the fourth day, he developed shock and seizures. On the fifth day, he died.
Serum tests for immunoglobulin G (IgG) and immunoglobulin M (IgM) in this patient were negative for YF on days 2-7. Reverse-transcriptase polymerase chain reaction (PCR) assay of serum samples obtained on days 4, 5, and 7 and a postmortem liver specimen demonstrated YF virus RNA.
International statistics
Each year, an estimated 200,000 cases of YF occur in Africa and South America combined, causing an estimated 30,000 deaths. The number of total cases reported to the WHO each year from Africa and South America ranges from hundreds to a few thousand. The true incidence is estimated to be at least 40 times higher than the reported incidence in Africa and 10 times higher than the reported incidence in South America. [6]
Underreporting of YF occurs because many cases are mild or asymptomatic, because cases occur in remote regions, because populations are moving or displaced, or because the public health infrastructure is minimal or nonexistent (particularly in Africa, where regional conflicts are ongoing).
YF has been reemerging since 1980, with more cases being reported now than in the late 1940s. This trend is evident in Africa, where the number of countries reporting cases and the number of small-scale outbreaks have increased, where large populations are losing immunity (as a consequence of the end of mass-prevention campaigns in the early 1960s), and where vectors are present in urban areas.
In addition, African urban populations are markedly increasing by 4.8% per year), populations are migrating (either voluntarily or as a result of force), and vaccine often cannot be procured because of the expense.
In South America, A aegypti is now present in urban areas. This mosquito is the primary vector of urban YF, breeding in domestic and peridomestic containers. In the past 30 years, A aegypti has reinfested most of the countries from which it had previously been eradicated.
By 2008, A aegypti was present in the southern United States, Central America, the Caribbean, most major urban centers in tropical South America, the Indian subcontinent, Southeast Asia, Oceania, and northeast Australia. A legitimate concern is that YF could be reintroduced into these areas at any time because of global travel and trade and because of migrating populations. Nevertheless, YF has never been reported to be endemic to Asia.
The CDC estimates that YF immunization of travelers to YF-endemic areas declined 50% from 1992 to 1998. Travel to such areas poses a threat to the unimmunized traveler. Each year, an estimated 9 million travelers from North America, Europe, and Asia travel to endemic countries. At least one third of these travelers are exposed to areas where active transmission is known or unknown to be occurring.
Since 1979, at least 9 cases of traveler-related YF have been reported. Seven individuals, all unimmunized, died. Of the 2 survivors, 1 had been immunized. All traveled to rural areas, where no epidemics were known to be occurring.
The overall risk to an unimmunized traveler in Africa who is entering an area with epidemic activity is 1:267 for YF illness and 1:1333 for YF death during a 2-week trip. If the area is undergoing a silent period (ie, if existing surveillance methods do not detect active transmission), the risk is 1:2000 for YF illness and 1:10,000 for YF death for a 2-week trip. In South America, the risk is estimated to be one tenth as high.
In West Africa, the most dangerous time of year is between July and October; in Brazil, it is between January and March. In South America, Iguazú Falls on the Argentine-Brazil border is not considered a high-risk destination, but the risk is not zero either. For example, in 1996 and 2001, risk increased because of epizootic expansion. This example illustrates how the status of YF transmission in endemic countries is constantly shifting and unpredictable.
Reemergence in South America
YF is reemerging in South America. The following 3 factors are contributing to this reemergence:
-
Reinvasion of A aegypti since 1980 secondary to reductions in mosquito-control measures
-
Juxtaposition of areas of jungle transmission with areas of A aegypti infestation, allowing the latter to become reinfected
-
Lack of YF vaccination in densely populated areas outside of the traditional jungle transmission zone, creating huge populations at risk
Between 2007 and 2009, YF cases in South America were reported outside the usual areas of risk in Argentina, Brazil, and Paraguay.
In Argentina, 5 cases of jungle YF (with 1 death) were reported in Misiones Province. To contain this outbreak, more than 1.4 million people in Argentina have received YF vaccine (0.4 million in Misiones Province).
In Brazil, 45 cases of jungle YF were reported, with 25 deaths among 4 states. Parts of the states of Sao Paulo and Minas Gerais reported cases outside their usual risk areas, and the state of Espirito Santo, which is on the coast and is not a usual risk area at all, reported cases; however, as of late 2008, it is no longer considered a risk area.
In December 2008, Rio Grande do Sul, which is also outside the usual reporting area, reported 18 cases, including 7 deaths. Since February 2009, the state of Sao Paulo has reported 25 YF cases, including 9 deaths. To contain this outbreak, YF vaccinations have been increased in the reporting states and increased among travelers to these states, although YF vaccination coverage is already extensive.
In Paraguay, the first cases of YF in more than 30 years were reported in 2008 (25 cases, 8 deaths). These cases were mostly jungle YF but may have included urban YF; the city of San Lorenzo alone reported 9 cases with 3 deaths, YF cases occurred in 3 departments (San Pedro, Central, and Caaguazú). To contain this outbreak, more than 1.2 million people in 15 departments of Paraguay were given YF vaccine.
In Argentina and Brazil, the human outbreaks were preceded by weeks with an epizootic in monkeys.
Age-, sex-, and race-related differences in incidence
YF has no known age predilection. However, most infections in endemic countries occur in persons younger than 15 years because this group contains the largest population of nonimmune individuals. Occupational or recreational exposure may be increased among children as well.
No sex predilection is known. Because of occupational exposure, more men than women are infected in jungles and forests. No racial predilection is known.
Prognosis
Mortality from the toxic form of YF ranges from 25% to 50%, but the overall mortality has been reported to be as low as 1%. The number of reported deaths from YF among travelers over the past 10 years has increased, and more can be expected unless YF vaccine is most appropriately used.
If death does not occur, recovery without sequelae is the rule. Morbidity is minimal unless complications develop from the toxic form of the disease. Recovery from YF confers life-long immunity to reinfection.
Indicators of poor prognosis include the following:
-
Early onset of bilirubinemia
-
Marked albuminuria
-
Prothrombin time increased by more than 25%
-
Severe hemorrhage
-
Shock
-
Intractable hiccough (hiccup)
Complications
Complications of YF include the following:
-
Organ failure - Liver (secondary to hepatic necrosis), kidney (secondary to parenchymal injury and acute tubular necrosis), heart (secondary to parenchymal injury)
-
Hemorrhagic diathesis - YF virus directly injures the liver, kidney, and heart; a hemorrhagic diathesis progressing to disseminated intravascular coagulation (DIC) is not uncommon in the toxic form of the disease, because of hepatic damage, thrombocytopenia, and perhaps other factors
-
Secondary infections, particularly bacterial pneumonia
Patient Education
Increased public awareness of YF is important. All travelers going to YF-endemic areas (eg, destinations in the tropics or developing countries) should at least see a health provider who is familiar with traveler’s health recommendations from public health agencies. One source of such recommendations is the Traveler’s Health page of the CDC.
Even if the WHO does not have a requirement for YF vaccination for travel to a country, a traveler may still be exposed and concern may be warranted.
-
This female Aedes aegypti mosquito is shown here after landing on a human host. The A aegypti mosquito is a known transmitter of both dengue fever and yellow fever. A aegypti is sometimes referred to as the yellow fever mosquito. The viruses are transferred to the host when bitten by a female mosquito. Image courtesy of the Centers for Disease Control and Prevention (CDC) and the World Health Organization (WHO).






