Background
Truncus arteriosus (TA) is an uncommon congenital cardiovascular anomaly that is characterized by a single arterial trunk arising from the normally formed ventricles by means of a single semilunar valve (ie, truncal valve). In the most common type, the pulmonary arteries originate from the common arterial trunk distal to the coronary arteries and proximal to the first brachiocephalic branch of the aortic arch. The common trunk typically straddles a defect in the outlet portion of the interventricular septum (ie, conal septum); however, in rare cases, it may originate almost completely from the right or left ventricle. In patients with a patent and normal caliber aortic arch, the ductus arteriosus is either absent or diminutive.
Embryology
The anomaly is thought to result from incomplete or failed septation of the embryonic truncus arteriosus, hence the persistence of the Latin term truncus arteriosus and its variants. Aortopulmonary and interventricular defects are believed to represent an abnormality of conotruncal septation. Because the common trunk originates from both the left and right ventricles, and pulmonary arteries arise directly from the common trunk, a ductus arteriosus is not required to support the fetal circulation.
Accordingly, an inverse relationship between the caliber of the ductus arteriosus (derived from the sixth branchial arch) and that of the distal portion of the aortic arch (derived from the fourth branchial arch) is typically present. Although the hemodynamic consequences of a common arterial outflow may predispose to the development of the fourth or the sixth arch (but not both), anomalous development of the arch system is likely a fundamental aspect of the morphogenetic anomalies that produce truncus arteriosus.
Anatomy
Pulmonary arteries may arise from the common trunk in one of several patterns, which are often used to classify subtypes of truncus arteriosus. Several classification schemes have been proposed, none of which is ideal.
The earliest classification, developed by Collett and Edwards in 1949, includes truncus arteriosus types I-IV, as follows [1] :
-
Truncus arteriosus type I is characterized by origin of a single pulmonary trunk from the left lateral aspect of the common trunk, with branching of the left and right pulmonary arteries from the pulmonary trunk.
-
Truncus arteriosus type II is characterized by separate but proximate origins of the left and right pulmonary arterial branches from the posterolateral aspect of the common arterial trunk.
-
In truncus arteriosus type III, the branch pulmonary arteries originate independently from the common arterial trunk or aortic arch, most often from the left and right lateral aspects of the trunk. This occasionally occurs with origin of one pulmonary artery from the underside of the aortic arch, usually from a ductus arteriosus.
-
Type IV truncus arteriosus, originally proposed by Collett and Edwards as a form of the lesion with neither pulmonary arterial branch arising from the common trunk, is now recognized to be a form of pulmonary atresia with ventricular septal defect rather than truncus arteriosus.
Collett and Edwards describe variations of each of these types.
In 1965, Van Praaghs proposed the other commonly cited classification scheme that also includes 4 primary types, as follows [2] :
-
Type A1 is identical to the type I of Collett and Edwards.
-
Type A2 includes Collett and Edwards type II and most cases of type III, namely those with separate origin of the branch pulmonary arteries from the left and right lateral aspects of the common trunk.
-
Type A3 includes cases with origin of one branch pulmonary artery (usually the right) from the common trunk, with pulmonary blood supply to the other lung provided either by a pulmonary artery arising from the aortic arch (a subtype of Collett and Edwards type III) or by systemic to pulmonary arterial collaterals.
-
Type A4 is defined not by the pattern of origin of branch pulmonary arteries, but rather by the coexistence of an interrupted aortic arch. In the vast majority of cases of type A4, which fall into the type I of Collett and Edwards, the pulmonary arteries arise as a single pulmonary trunk that then branches. In any of these patterns, intrinsic stenosis, hypoplasia, or both may be present in one or both branch pulmonary arteries, which may have an effect on management and outcome.
The Van Praagh scheme is combined with Collett and Edwards types in the image below.
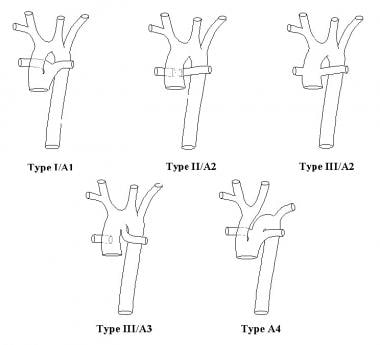 Anatomic subtypes of truncus arteriosus (TA), according to the classification systems of both Collett and Edwards (I, II, III) and the Van Praaghs (A1, A2, A3, A4).
Anatomic subtypes of truncus arteriosus (TA), according to the classification systems of both Collett and Edwards (I, II, III) and the Van Praaghs (A1, A2, A3, A4).
Associated cardiovascular anomalies
Various abnormalities may be associated with truncus arteriosus, some of which may have an impact on management and outcome.
Structural abnormalities of the truncal valve, including dysplastic and supernumerary leaflets, are frequently observed, and significant stenosis or regurgitation (moderate or severe) through the truncal valve may be present in 20% or more patients.
Similarly, proximal coronary arteries are abnormal in many patients, with a single coronary artery and an intramural course as the most important variations.
The other major anomaly associated with truncus arteriosus in a substantial portion of cases is interruption of the aortic arch, which almost always occurs between the left common carotid and subclavian arteries.
Other relatively common but minor associations include right aortic arch, left superior caval vein, aberrant subclavian artery, and atrial septal defect. In addition to these defects found in the usual spectrum of truncus arteriosus, several other major but rare associated anomalies are reported, including complete atrioventricular septal defect, double aortic arch, and various forms of functionally univentricular heart.
Sepsis is probably the most important noncardiac problem in the differential diagnosis of neonates with truncus arteriosus, as well as other forms of complex congenital heart disease. Young infants with truncus arteriosus frequently present in shock because of high output heart failure with significant pulmonary overcirculation. This scenario may resemble the presentation of neonatal sepsis, especially when the ratio of pulmonary-to-systemic blood flow is sufficiently high that the patient is not cyanotic.
Pathophysiology
Pathophysiology of truncus arteriosus is typified by pulmonary overcirculation and systemic ventricular volume overload. Outflow from both ventricles is directed into the common arterial trunk. Pulmonary blood flow is derived from this combined ventricular output, and its magnitude depends on the ratio of resistances to flow in the pulmonary and systemic vascular beds. Because of the mixing (although not complete) of left and right ventricular output that occurs primarily during systole and at the level of the common arterial trunk, subnormal systemic arterial oxygen saturation may occur, although it usually is not significant enough to result in clinical cyanosis. Similarly, because the systemic and pulmonary circulations are essentially in parallel, pulmonary blood flow typically is at least 3-fold higher than systemic blood flow, with pulmonary overcirculation and increased myocardial work that results in increased resting oxygen demand and decreased metabolic reserve.
Etiology
As with most forms of congenital heart disease, the causes of truncus arteriosus are unknown. In experimental animal models, truncus arteriosus has been linked to abnormal development of cells from the neural crest that normally inhabit the outflow region of the developing heart. This is thought to be an important etiologic factor in at least some cases of human truncus arteriosus also.
As with various other congenital cardiac anomalies of the conotruncal region, a substantial number of patients with truncus arteriosus (approximately 30-40%) have microdeletions within chromosome band 22q11.2, which contains a number of characterized genes. This particular type of chromosomal deletion is thought to affect migration or development of cardiac neural crest cells and may contribute to the pathogenesis of truncus arteriosus in certain cases.
Patients with truncus arteriosus and anomalies of the branch pulmonary arteries, such as stenosis or separate origin from the undersurface of the aortic arch, may have a higher incidence of association with band 22q11 deletion. Other specific features of truncus arteriosus that may be related to chromosomal deletion have yet to be characterized. [3]
The specific gene product or products responsible for cardiovascular anomalies in individuals with a 22q11 deletion has not been identified definitively in humans, although one of the genes in the 22q11.2 band, TBX1, has been shown to be involved in pharyngeal arch and conotruncal development. Extensive research regarding truncus arteriosus and band 22q11 association is being conducted.
For the most part, other factors that may cause truncus arteriosus in humans have not been clearly identified, although potential associations have been suggested, such as the following:
-
Other sporadic chromosomal and genetic abnormalities have been reported in humans with truncus arteriosus, including duplication of chromosome arm 8q and mutation of the NKX2.6 and GATA6 genes. [4]
-
Several other genes have been associated with truncus arteriosus in transgenic mouse models, including Tbx20, ALK2, Cited2, and Semaphorin 3c, but so far these genes have not been implicated in human truncus arteriosus.
-
Although certain teratogens (eg, retinoic acid, bis-diamine) have been found to predispose to truncus arteriosus in animal models, no evidence suggests that these or others contribute importantly to this anomaly in humans.
DiGeorge syndrome or velocardiofacial syndrome, often included together as variations of CATCH-22 syndrome, are present in approximately 30-35% of patients with truncus arteriosus; most of these patients have deletions in band 22q11.
The most common noncardiac anomalies in patients with truncus arteriosus are those typically found in association with CATCH-22 syndrome, such as velopharyngeal insufficiency, cleft palate, and thymic and parathyroid dysfunction.
Other noncardiac anomalies found sporadically in patients with truncus arteriosus include renal abnormalities, vertebral and rib anomalies, and anomalies of the alimentary tract.
Epidemiology
United States data
Truncus arteriosus represents 1-2% of congenital heart defects in liveborn infants. Based on an estimated incidence of congenital heart disease of 6-8 per 1,000 liveborn children, truncus arteriosus occurs in approximately 5-15 of 100,000 live births. Among aborted fetuses and stillborn infants with cardiovascular anomalies, truncus arteriosus represents almost 5% of defects.
A population-based review of all cases of live births from 1999 to 2008 identified as having severe congenital heart disease from the Nationwide Inpatient Sample (NIS) database indicated a decrease of the conditions over the study period. [7] There was a significant decreased incidence of truncus arteriosus as well as tetralogy of Fallot, pulmonary atresia, and hypoplastic left heart syndrome; however, these trends varied with sociodemographic factors. The investigators suggested a possible reason for the decreasing prevalence trend was the increased numbers of terminated fetuses with prenatally diagnosed congenital heart disease. [7]
International data
No significant difference in the incidence of truncus arteriosus is noted among those born in the United States compared with other countries.
However, a Canadian longitudinal study (1983-2010) of all individual with congenital heart disease noted a more than 50% increase of severe and other congenital heart disease after the year 2000, with adults comprising two thirds of the cases by 2010. [8] The prevalence of congenital heart disease in the first year of life between 1998 and 2005 was 8.21 per 1000 live births; in 2010, the overall prevalence was 13.11 per 1000 in children and 6.12 per 1000 in adults. A temporal increase in prevalence of congenital heart disease and severe congenital heart disease was noted for children and adults. [8]
Race-, sex-, and age-related demographics
Based on limited data, no racial predilection is apparent.
Although many series report a slight male predominance, no significant predilection based on sex is apparent.
Truncus arteriosus is a congenital anomaly that is present from early in embryonic gestation. Currently, truncus arteriosus is diagnosed using prenatal ultrasonography in a small percentage of patients. Among patients diagnosed after birth, the median age at presentation is generally a few days, which is significantly earlier than was the case 20 or more years ago. Occasionally, patients are not diagnosed until later in infancy, childhood, or even adulthood, although such cases are exceedingly rare in the United States and Europe.
Prognosis
Among patients surviving the early postoperative period, prognosis is generally very good. Few published long-term follow-up data are available on patients undergoing repair in the neonatal and early infant periods because this management strategy came into widespread application in the mid to late 1980s. Moreover, techniques of myocardial protection and perioperative management have changed dramatically even within this period; thus, existing data, limited as they may be, are still likely to underestimate outcome in contemporary patients.
Although late mortality among patients undergoing early repair is minimal, a substantial proportion of premature deaths among such patients are likely to be related to reinterventions. Because the right ventricular outflow tract is usually reconstructed with a nonviable conduit, which does not grow along with the patient, reinterventions for conduit replacement, revision, or dilation are essentially inevitable. In a series following infants younger than 4 months with surgically repaired truncus arteriosus, freedom from conduit-related reintervention was less than 50% at 5 years and less than 10% at 10 years.
Patients who have the conduit replaced earlier in life often require at least one subsequent intervention on the right ventricular outflow tract. Reintervention for truncal valve regurgitation (often within the first year after repair) or for branch pulmonary arterial stenosis is also required in a substantial number of patients.
At major centers in North America, survival to hospital discharge after complete repair of truncus arteriosus is approximately 90-95%. Prognosis appears somewhat less favorable for patients with complicating associated conditions, such as severe truncal valve regurgitation of interruption of the aortic arch. Significant perioperative morbidity is uncommon and includes issues common to many forms of complex congenital heart disease, such as transient arrhythmias, low cardiac output, and sequelae of cardiopulmonary bypass.
Morbidity/mortality
The natural history of truncus arteriosus without surgical intervention is not well characterized. In numerous earlier series, the median age at death without surgery ranged from 2 weeks to 3 months, with almost 100% mortality by age 1 year. Cases of patients surviving into adulthood with unrepaired truncus arteriosus are reported, but they are extremely uncommon. Cause of death in unrepaired patients is usually cardiac arrest or multiple organ failure in the face of systemic perfusion that is inadequate to meet the body's metabolic demands; progressive metabolic acidosis and myocardial dysfunction results.
Currently, for patients undergoing complete repair in the neonatal or early infant periods, early postoperative mortality is generally less than 10%. This represents a substantial improvement from earlier eras; as recently as 20 years ago, the early mortality rate after complete repair was higher than 25% in most series. Among patients surviving the initial postoperative period, the survival rate at a 10- to 20-year follow-up is higher than 80%, with most deaths resulting from sequelae of late repair (pulmonary vascular obstructive disease), reinterventions, or residual/recurrent physiologic abnormalities.
Although rarely used today, surgical palliation by banding of the pulmonary artery to protect the pulmonary vascular bed was a frequently used strategy until the 1970s and early 1980s. This practice resulted in only minor improvement in the natural history of the disease, with substantial early and intermediate mortality rates.
-
Anatomic subtypes of truncus arteriosus (TA), according to the classification systems of both Collett and Edwards (I, II, III) and the Van Praaghs (A1, A2, A3, A4).
-
Pathologic specimen with truncus arteriosus (TA), viewed through the opened right ventricle and truncal valve. The common trunk (CT) can be seen giving off the ascending aorta (AA) as well as the left (LPA) and right (RPA) pulmonary arteries. The truncal valve straddles the ventricular septal defect (VSD). The tricuspid valve (TV) also is labeled. Photograph courtesy of Robert H. Anderson, MD.
-
Pathologic specimen with truncus arteriosus (TA) and interruption of the aortic arch between the left (L) common carotid (CCA) and subclavian (SCA) arteries, viewed from the anterior aspect. The common trunk (CT) is seen arising from the ventricular mass, including the right ventricular (RV) infundibulum. Pulmonary arteries arise as a single trunk from the leftward aspect of the common trunk, which then divides into left and right branches (not shown) and the arterial duct (DA), which continues into the descending aorta, from which the left subclavian artery arises. The ascending aorta (AA), which supplies only the right (R) and left common carotid arteries (the right subclavian artery, which arises anomalously as the last brachiocephalic branch, is not shown), continues from the rightward aspect of the common trunk and is much smaller than in patients without an interrupted arch. RA=right atrial appendage. Photograph courtesy of Robert H. Anderson, MD.
-
Echocardiographic images of truncus arteriosus (TA). The top image is from the subcostal coronal window (SC COR) and shows the common trunk (TR) arising from the left ventricle (LV), overriding the interventricular septum. The common trunk branches into the pulmonary trunk and the ascending aorta (AO). The left pulmonary artery (LPA) may be seen branching from the pulmonary trunk. RA=right atrium; RPA=right pulmonary artery. In the bottom image, which is from the suprasternal notch sagittal window, the truncal origin and course of the pulmonary trunk and left pulmonary artery can be appreciated. DAO=descending aorta; IV=innominate vein; LA=left atrium.







