Mazur-Melewska K, Mania A, Figlerowicz M, Kemnitz P, Sluzewski W, Michalak M. The influence of age on a clinical presentation of Toxocara spp. infection in children. Ann Agric Environ Med. 2012. 19(2):233-6. [QxMD MEDLINE Link].
Knopp S, Steinmann P, Keiser J, Utzinger J. Nematode infections: soil-transmitted helminths and trichinella. Infect Dis Clin North Am. 2012 Jun. 26(2):341-58. [QxMD MEDLINE Link].
Maruyama H, Nawa Y, Noda S, et al. An outbreak of visceral larva migrans due to Ascaris suum in Kyushu, Japan. Lancet. 1996 Jun 22. 347(9017):1766-7. [QxMD MEDLINE Link].
Congdon P, Lloyd P. Toxocara infection in the United States: the relevance of poverty, geography and demography as risk factors, and implications for estimating county prevalence. Int J Public Health. 2011 Feb. 56(1):15-24. [QxMD MEDLINE Link].
Red Book. Toxocariasis. 2009 Report of the Committee on Infectious Diseases. 28th ed. American Academy of Pediatrics. 2009. 666-67.
Turrientes MC, Perez de Ayala A, Norman F, Navarro M, Perez-Molina JA, Rodriquez-Ferrer M, et al. Visceral larva migrans in immigrants from latin america. Emerg Infect Dis. 2011 Jul. 17(7):1263-5. [QxMD MEDLINE Link].
Nkouawa A, Sako Y, Itoh S, et al. Serological studies of neurologic helminthic infections in rural areas of southwest cameroon: toxocariasis, cysticercosis and paragonimiasis. PLoS Negl Trop Dis. 2010 Jul 6. 4(7):e732. [QxMD MEDLINE Link]. [Full Text].
Colli CM, Rubinsky-Elefant G, Paludo ML, et al. Serological, clinical and epidemiological evaluation of toxocariasis in urban areas of south Brazil. Rev Inst Med Trop Sao Paulo. 2010 Apr. 52(2):69-74. [QxMD MEDLINE Link].
Espinoza YA, Huapaya PE, Roldan WH, et al. Seroprevalence of human toxocariasis in Andean communities from the Northeast of Lima, Peru. Rev Inst Med Trop Sao Paulo. 2010 Feb. 52(1):31-6. [QxMD MEDLINE Link].
Liao CW, Sukati H, D'Lamini P, et al. Seroprevalence of Toxocara canis infection among children in Swaziland, southern Africa. Ann Trop Med Parasitol. 2010 Jan. 104(1):73-80. [QxMD MEDLINE Link].
Akdemir C. Visceral larva migrans among children in Kütahya (Turkey) and an evaluation of playgrounds for T. canis eggs. Turk J Pediatr. 2010 Mar-Apr. 52(2):158-62. [QxMD MEDLINE Link].
Elshazly AM, Attia G, El-Ghareeb AS, Belal US. Clinical varieties of Toxocariasis canis in Children's Hospital, Mansoura University: is it an underestimated problem?. J Egypt Soc Parasitol. 2011 Aug. 41(2):263-74. [QxMD MEDLINE Link].
Kanobana K, Vereecken K, Junco Diaz R, Sariego I, Rojas L, Bonet Gorbea M, et al. Toxocara seropositivity, atopy and asthma: a study in Cuban schoolchildren. Trop Med Int Health. 2013 Feb 8. [QxMD MEDLINE Link].
Sariego I, Kanobana K, Junco R, Vereecken K, Núñez FA, Polman K, et al. Frequency of antibodies to Toxocara in Cuban schoolchildren. Trop Med Int Health. 2012 Jun. 17(6):711-4. [QxMD MEDLINE Link].
Pinelli E, Herremans T, Harms MG, Hoek D, Kortbeek LM. Toxocara and Ascaris seropositivity among patients suspected of visceral and ocular larva migrans in the Netherlands: trends from 1998 to 2009. Eur J Clin Microbiol Infect Dis. 2011 Jul. 30(7):873-9. [QxMD MEDLINE Link].
Rostami A, Riahi SM, Holland CV, et al. Seroprevalence estimates for toxocariasis in people worldwide: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2019 Dec. 13 (12):e0007809. [QxMD MEDLINE Link].
Lynch NR, Eddy K, Hodgen AN, et al. Seroprevalence of Toxocara canis infection in tropical Venezuela. Trans R Soc Trop Med Hyg. 1988. 82(2):275-81. [QxMD MEDLINE Link].
Cassenote AJ, Lima AR, Pinto Neto JM, Rubinsky-Elefant G. Seroprevalence and modifiable risk factors for Toxocara spp. in Brazilian schoolchildren. PLoS Negl Trop Dis. 2014 May. 8(5):e2830. [QxMD MEDLINE Link]. [Full Text].
Oski FA, et al. Protozoan Parasites. Toxocariasis. Principles and Practices of Pediatrics. 2nd edition. Philadelphia, PA: JB Lippincott; 1994. 1405-08.
Hurni MA, Gerbig AW, Braathen LR, Hunziker T. Toxocariasis and Wells' syndrome: a causal relationship?. Dermatology. 1997. 195(4):325-8. [QxMD MEDLINE Link].
Wolfrom E, Chene G, Lejoly-Boisseau H, Beylot C, Geniaux M, Taieb A. [Chronic urticaria and toxocara canis infection. A case-control study]. Ann Dermatol Venereol. 1996. 123(4):240-6. [QxMD MEDLINE Link].
Bachmeyer C, Lamarque G, Morariu R, et al. Visceral larva migrans mimicking lymphoma. Chest. 2003 Apr. 123(4):1296-7. [QxMD MEDLINE Link].
Amir J, Harel L, Eidlitz-Markus T, Varsano I. Lymphedema as a presenting sign of toxocariasis. Infection. 1995 Nov-Dec. 23(6):389-90. [QxMD MEDLINE Link].
Cruz AT, Franklin GY, Kaplan SL. Toxocariasis Causing Eosinophilic Ascites. Pediatr Infect Dis J. 2008 Apr 23. 27(6):563-4. [QxMD MEDLINE Link].
Hamidou MA, Gueglio B, Cassagneau E, et al. Henoch-Schonlein purpura associated with Toxocara canis infection. J Rheumatol. 1999 Feb. 26(2):443-5. [QxMD MEDLINE Link].
De Cock C, Lemaitre J, Deuvaert FE. Loeffler endomyocarditis: a clinical presentation as right ventricular tumor. J Heart Valve Dis. 1998 Nov. 7(6):668-71. [QxMD MEDLINE Link].
Herry I, Philippe B, Hennequin C, et al. Acute life-threatening toxocaral tamponade. Chest. 1997 Dec. 112(6):1692-3. [QxMD MEDLINE Link]. [Full Text].
Tobin EH, Zhang J, Maton B. Meningoencephalitis and visceral larva migrans in a woman with intense exposure to cats. Infect Dis Clin Pract. May, 2011. 19(3):221-2.
Salvador S, Ribeiro R, Winckler MI, Ohlweiler L, Riesgo R. Pediatric neurotoxocariasis with concomitant cerebral, cerebellar, and peripheral nervous system involvement: case report and review of the literature. J Pediatr (Rio J). 2010 Nov-Dec. 86(6):531-4. [QxMD MEDLINE Link].
Centers for Disease Control and Prevention. Ocular toxocariasis--United States, 2009-2010. MMWR Morb Mortal Wkly Rep. 2011 Jun 10. 60(22):734-6. [QxMD MEDLINE Link].
Ocular toxocariasis--United States, 2009-2010. MMWR Morb Mortal Wkly Rep. 2011 Jun 10. 60(22):734-6. [QxMD MEDLINE Link].
Oski FA, et al. Ambulatory Pediatrics. Endophthalmitis. Principles and Practices of Pediatrics. 2nd edition. Philadelphia, PA: JB Lippincott; 1994. 888.
Stewart JM, Cubillan LD, Cunningham ET. Prevalence, clinical features, and causes of vision loss among patients with ocular toxocariasis. Retina. 2005 Dec. 25(8):1005-13. [QxMD MEDLINE Link].
Woodhall D. Neglected Parasitic Infections: Toxocariasis. Centers for Disease Control and Prevention. Available at http://www2c.cdc.gov/podcasts/media/pdf/COCA_Toxocariasis.pdf. Accessed: February 4, 2015.
Abo-Shehada MN, Sharif L, el-Sukhon SN, et al. Seroprevalence of Toxocara canis antibodies in humans in northern Jordan. J Helminthol. 1992 Mar. 66(1):75-8. [QxMD MEDLINE Link].
Agudelo C, Villareal E, Caceres E, et al. Human and dogs Toxocara canis infection in a poor neighborhood in Bogota. Mem Inst Oswaldo Cruz. 1990 Jan-Mar. 85(1):75-8. [QxMD MEDLINE Link].
Akao N, Ohta N. Toxocariasis in Japan. Parasitol Int. 2007 Jun. 56(2):87-93. [QxMD MEDLINE Link].
Altcheh J, Nallar M, Conca M, et al. [Toxocariasis: clinical and laboratory features in 54 patients]. An Pediatr (Barc). 2003 May. 58(5):425-31. [QxMD MEDLINE Link].
American Academy of Pediatrics. Toxocariasis (Visceral Larva Migrans, Ocular Larva Migrans). Red Book: 2009 Report of the Committee on Infectious Diseases. 28th. Elk Grove Village, IL: American Academy of Pediatrics; 2009. 666-7.
Arango CA. Visceral larva migrans and the hypereosinophilia syndrome. South Med J. 1998 Sep. 91(9):882-3. [QxMD MEDLINE Link].
Arpino C, Gattinara GC, Piergili D, Curatolo P. Toxocara infection and epilepsy in children: a case-control study. Epilepsia. 1990 Jan-Feb. 31(1):33-6. [QxMD MEDLINE Link].
Ashwath ML, Robinson DR, Katner HP. A presumptive case of toxocariasis associated with eosinophilic pleural effusion: case report and literature review. Am J Trop Med Hyg. 2004 Dec. 71(6):764. [QxMD MEDLINE Link]. [Full Text].
Toxocariasis. Spector JM, Gibson TE. Atlas of Pediatrics in the Tropics and Resource-Limited Settings. Elk Grove Village, IL: American Academy of Pediatrics; 2009. 249-251.
Baldisserotto M, Conchin CF, Soares Mda G, et al. Ultrasound findings in children with toxocariasis: report on 18 cases. Pediatr Radiol. 1999 May. 29(5):316-9. [QxMD MEDLINE Link].
Bass JL, Mehta KA, Glickman LT, et al. Asymptomatic toxocariasis in children. A prospective study and treatment trial. Clin Pediatr (Phila). 1987 Sep. 26(9):441-6. [QxMD MEDLINE Link].
Bede O, Szénási Z, Danka J, Gyurkovits K, Nagy D. Toxocariasis associated with chronic cough in childhood: a longitudinal study in Hungary. J Helminthol. 2008 Dec. 82(4):357-63. [QxMD MEDLINE Link].
Beiran I, Cochavi O, Miller B. "Silent" ocular toxocariasis. Eur J Ophthalmol. 1998 Jul-Sep. 8(3):195-6. [QxMD MEDLINE Link].
Buijs J, Borsboom G, van Gemund JJ, et al. Toxocara seroprevalence in 5-year-old elementary schoolchildren: relation with allergic asthma. Am J Epidemiol. 1994 Nov 1. 140(9):839-47. [QxMD MEDLINE Link].
Carvalho EA, Rocha RL. Toxocariasis: visceral larva migrans in children. J Pediatr (Rio J). 2011 Mar-Apr. 87(2):100-10. [QxMD MEDLINE Link].
Chang S, Lim JH, Choi D, et al. Hepatic visceral larva migrans of Toxocara canis: CT and sonographic findings. AJR Am J Roentgenol. 2006 Dec. 187(6):W622-9. [QxMD MEDLINE Link].
Cianferoni A, Schneider L, Schantz PM, et al. Visceral larva migrans associated with earthworm ingestion: clinical evolution in an adolescent patient. Pediatrics. 2006 Feb. 117(2):e336-9. [QxMD MEDLINE Link].
Cilla G, Perez-Trallero E, Gutierrez C, et al. Seroprevalence of Toxocara infection in middle-class and disadvantaged children in northern Spain (Gipuzkoa, Basque Country). Eur J Epidemiol. 1996 Oct. 12(5):541-3. [QxMD MEDLINE Link].
Dauriac-Le Masson V, Chochon F, Demeret S, Pierrot-Deseilligny C. Toxocara canis meningomyelitis. J Neurol. 2005 Oct. 252(10):1267-8. [QxMD MEDLINE Link].
Despommier D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003 Apr. 16(2):265-72. [QxMD MEDLINE Link].
Eberhard ML, Alfano E. Adult Toxocara cati infections in U.S. children: report of four cases. Am J Trop Med Hyg. 1998 Sep. 59(3):404-6. [QxMD MEDLINE Link]. [Full Text].
Eberhardt O, Bialek R, Nagele T, Dichgans J. Eosinophilic meningomyelitis in toxocariasis: case report and review of the literature. Clin Neurol Neurosurg. 2005 Aug. 107(5):432-8. [QxMD MEDLINE Link].
Elefant GR, Shimizu SH, Sanchez MC, et al. A serological follow-up of toxocariasis patients after chemotherapy based on the detection of IgG, IgA, and IgE antibodies by enzyme-linked immunosorbent assay. J Clin Lab Anal. 20(4):164-72. [QxMD MEDLINE Link].
Fenoy S, Cuellar C, Guillen JL. Serological evidence of toxocariasis in patients from Spain with a clinical suspicion of visceral larva migrans. J Helminthol. 1997 Mar. 71(1):9-12. [QxMD MEDLINE Link].
Fenoy S, Cuellar C, Guillen JL. Seroprevalence of toxocariasis in children and adults in Madrid and Tenerife, Spain. J Helminthol. 1996 Jun. 70(2):109-13. [QxMD MEDLINE Link].
Fortenberry JD, Kenney RD, Younger J. Visceral larva migrans producing static encephalopathy in an infant. Pediatr Infect Dis J. 1991 May. 10(5):403-6. [QxMD MEDLINE Link].
Frazier M, Anderson ML, Sophocleous S. Treatment of ocular toxocariasis with albendezole: a case report. Optometry. 2009 Apr. 80(4):175-80. [QxMD MEDLINE Link].
Gavignet B, Piarroux R, Aubin F, Millon L, Humbert P. Cutaneous manifestations of human toxocariasis. J Am Acad Dermatol. 2008 Dec. 59(6):1031-42. [QxMD MEDLINE Link].
Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981. 3:230-50. [QxMD MEDLINE Link].
Good B, Holland CV, Taylor MR, et al. Ocular toxocariasis in schoolchildren. Clin Infect Dis. 2004 Jul 15. 39(2):173-8. [QxMD MEDLINE Link].
Gotistein B, Piarroux R. Current trends in tissue-affecting helminths. Parasite. 2008 Sep. 15(3):291-8. [QxMD MEDLINE Link].
Gould IM, Newell S, Green SH, George RH. Toxocariasis and eosinophilic meningitis. Br Med J (Clin Res Ed). 1985 Nov 2. 291(6504):1239-40. [QxMD MEDLINE Link].
Graeff-Teixeira C, da Silva AC, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009 Apr. 22(2):322-48, Table of Contents. [QxMD MEDLINE Link].
Guneratne R, Mendis D, Bandara T, Fernando SD. Toxoplasma, toxocara and tuberculosis co-infection in a four year old child. BMC Pediatr. 2011 May 26. 11:44. [QxMD MEDLINE Link]. [Full Text].
Havasiova K, Dubinsky P, Stefancikova A. A seroepidemiological study of human Toxocara infection in the Slovak Republic. J Helminthol. 1993 Dec. 67(4):291-6. [QxMD MEDLINE Link].
Hayashi K, Tahara H, Yamashita K, et al. Hepatic imaging studies on patients with visceral larva migrans due to probable Ascaris suum infection. Abdom Imaging. 1999 Sep-Oct. 24(5):465-9. [QxMD MEDLINE Link].
Heldrich FJ, Garg PP. Eosinophilic meningitis. Md Med J. 1988 Feb. 37(2):138-40. [QxMD MEDLINE Link].
Hill IR, Denham DA, Scholtz CL. Toxocara canis larvae in the brain of a British child. Trans R Soc Trop Med Hyg. 1985. 79(3):351-4. [QxMD MEDLINE Link].
Humbert P, Niezborala M, Salembier R, et al. Skin manifestations associated with toxocariasis: a case-control study. Dermatology. 2000. 201(3):230-4. [QxMD MEDLINE Link].
Iddawela RD, Rajapakse RP, Perera NA, Agatsuma T. Characterization of a Toxocara canis species-specific excretory-secretory antigen (TcES-57) and development of a double sandwich ELISA for diagnosis of visceral larva migrans. Korean J Parasitol. 2007 Mar. 45(1):19-26. [QxMD MEDLINE Link].
Inan M, Sakru N, Vatansever U, Bilgi S. Visceral larva migrans presenting as acute abdomen in a child. J Pediatr Surg. 41(3):e7-9. [QxMD MEDLINE Link].
Inatomi Y, Murakami T, Tokunaga M, et al. Encephalopathy caused by visceral larva migrans due to Ascaris suum. J Neurol Sci. 1999 Apr 1. 164(2):195-9. [QxMD MEDLINE Link].
Jain R, Sawhney S, Bhargava DK, et al. Hepatic granulomas due to visceral larva migrans in adults: appearance on US and MRI. Abdom Imaging. 1994 May-Jun. 19(3):253-6. [QxMD MEDLINE Link].
Jeanfaivre T, Cimon B, Tolstuchow N, et al. Pleural effusion and toxocariasis. Thorax. 1996 Jan. 51(1):106-7. [QxMD MEDLINE Link].
Kaushik SP, Hurwitz M, McDonald C, Pavli P. Toxocara canis infection and granulomatous hepatitis. Am J Gastroenterol. 1997 Jul. 92(7):1223-5. [QxMD MEDLINE Link].
Kayes SG. Human toxocariasis and the visceral larva migrans syndrome: correlative immunopathology. Chem Immunol. 1997. 66:99-124. [QxMD MEDLINE Link].
Kraus A, Valencia X, Cabral AR, de la Vega G. Visceral larva migrans mimicking rheumatic diseases. J Rheumatol. 1995 Mar. 22(3):497-500. [QxMD MEDLINE Link].
Krcmery V Jr, Gould I, Sobota K, Spanik S. Two cases of disseminated toxocariasis in compromised hosts successfully treated with mebendazole. Chemotherapy. 1992. 38(5):367-8. [QxMD MEDLINE Link].
Kumar J, Kimm J. MR in Toxocara canis myelopathy. AJNR Am J Neuroradiol. 1994 Nov. 15(10):1918-20. [QxMD MEDLINE Link].
Lassmann B, Tsigrelis C, Virk A. 33-year-old woman with marked eosinophilia. Mayo Clin Proc. 2007 Jan. 82(1):103-6. [QxMD MEDLINE Link].
Li MW, Lin RQ, Song HQ, Wu XY, Zhu XQ. The complete mitochondrial genomes for three Toxocara species of human and animal health significance. BMC Genomics. 2008 May 16. 9:224. [QxMD MEDLINE Link].
Lim JH. Toxocariasis of the liver: visceral larva migrans. Abdom Imaging. 2008 Mar-Apr. 33(2):151-6. [QxMD MEDLINE Link].
Ljungstrom I. Toxocara canis. Akuffo H, Linder E, Ljungstrom I, Wahlgren M. Parasites of the Colder Climates. London and New York: Taylor & Francis; 2003. 187-194.
Magnaval JF, Galindo V, Glickman LT, Clanet M. Human Toxocara infection of the central nervous system and neurological disorders: a case-control study. Parasitology. 1997 Nov. 115(Pt 5):537-43. [QxMD MEDLINE Link].
Matos M de F, Militao DN, Brum MA, et al. Presence of anti-Toxocara antibodies in children selected at Hospital Universitario, Campo Grande, MS, Brazil. Rev Inst Med Trop Sao Paulo. 1997 Jan-Feb. 39(1):49-50. [QxMD MEDLINE Link].
Matsuki Y, Fujii T, Nakamura-Uchiyama F, et al. Toxocariasis presenting with multiple effusions in the pericardial space, thoracic cavity, and Morrison's pouch. Intern Med. 2007. 46(12):913-4. [QxMD MEDLINE Link].
Mikhael NZ, Montpetit VJ, Orizaga M, et al. Toxocara canis infestation with encephalitis. Can J Neurol Sci. 1974 May. 1(2):114-20. [QxMD MEDLINE Link].
Mimoso MG, Pereira MC, Estevao MH, et al. Eosinophilic meningoencephalitis due to Toxocara canis. Eur J Pediatr. 1993 Sep. 152(9):783-4. [QxMD MEDLINE Link].
Moiyadi A, Mahadevan A, Anandh B, et al. Visceral larva migrans presenting as multiple intracranial and intraspinal abscesses. Neuropathology. 2007 Aug. 27(4):371-4. [QxMD MEDLINE Link].
Monsel G, Caumes E. Recent developments in dermatological syndromes in returning travelers. Curr Opin Infect Dis. 2008 Oct. 21(5):495-9. [QxMD MEDLINE Link].
Montalvo AM, Espino AM, Escalante G, Finlay CM. [Study of the seroprevalence of toxocariasis in an infantile population in the City of Havana]. Rev Cubana Med Trop. 1994. 46(3):156-8. [QxMD MEDLINE Link].
Moreira-Silva SF, Leao ME, Mendonca HF, Pereira FE. Prevalence of anti-Toxocara antibodies in a random sample of inpatients at a children's hospital in Vitoria, Espirito Santo, Brazil. Rev Inst Med Trop Sao Paulo. 1998 Jul-Aug. 40(4):259-61. [QxMD MEDLINE Link].
Murray MG, Bahna SL. Allergy consult for eosinophilia in an infant. Allergy Asthma Proc. 2012 Jul-Aug. 33(4):370-3. [QxMD MEDLINE Link].
Nathwani D, Laing RB, Currie PF. Covert toxocariasis--a cause of recurrent abdominal pain in childhood. Br J Clin Pract. 1992 Winter. 46(4):271. [QxMD MEDLINE Link].
Nelson S, Greene T, Ernhart CB. Toxocara canis infection in preschool age children: risk factors and the cognitive development of preschool children. Neurotoxicol Teratol. 1996 Mar-Apr. 18(2):167-74. [QxMD MEDLINE Link].
Ota KV, Dimaras H, Héon E, Babyn PS, Yau YC, Read S, et al. Toxocariasis mimicking liver, lung, and spinal cord metastases from retinoblastoma. Pediatr Infect Dis J. 2009 Mar. 28(3):252-4. [QxMD MEDLINE Link].
Oteifa NM, Moustafa MA, Elgozamy BM. Toxocariasis as a possible cause of allergic diseases in children. J Egypt Soc Parasitol. 1998 Aug. 28(2):365-72. [QxMD MEDLINE Link].
Patel H, Goldstein D. Pediatric uveitis. Pediatr Clin North Am. 2003 Feb. 50(1):125-36. [QxMD MEDLINE Link].
Petithory JC. [Immunologic diagnosis of ocular larva migrans syndrome]. Ophtalmologie. 1990 May-Jun. 4(3):298-300. [QxMD MEDLINE Link].
Rai SK, Uga S, Ono K, et al. Seroepidemiological study of Toxocara infection in Nepal. Southeast Asian J Trop Med Public Health. 1996 Jun. 27(2):286-90. [QxMD MEDLINE Link].
Rayes AA, Lambertucci JR. Visceral larva migrans and pyogenic liver abscess. Am J Gastroenterol. 1999 Apr. 94(4):1116. [QxMD MEDLINE Link].
Reilly A, Becker J, Meyer J, Rackoff W. Hypereosinophilia [clinical conference]. Med Pediatr Oncol. 1992. 20(3):232-9. [QxMD MEDLINE Link].
Roig J, Romeu J, Riera C, et al. Acute eosinophilic pneumonia due to toxocariasis with bronchoalveolar lavage findings. Chest. 1992 Jul. 102(1):294-6. [QxMD MEDLINE Link].
Roldán WH, Espinoza YA. Evaluation of an enzyme-linked immunoelectrotransfer blot test for the confirmatory serodiagnosis of human toxocariasis. Mem Inst Oswaldo Cruz. 2009 May. 104(3):411-8. [QxMD MEDLINE Link].
Romeu J, Roig J, Bada JL, et al. Adult human toxocariasis acquired by eating raw snails. J Infect Dis. 1991 Aug. 164(2):438. [QxMD MEDLINE Link].
Sabrosa NA, de Souza EC. Nematode infections of the eye: toxocariasis and diffuse unilateral subacute neuroretinitis. Curr Opin Ophthalmol. 2001 Dec. 12(6):450-4. [QxMD MEDLINE Link].
Sabrosa NA, Zajdenweber M. Nematode infections of the eye: toxocariasis, onchocerciasis, diffuse unilateral subacute neuroretinitis, and cysticercosis. Ophthalmol Clin North Am. 2002 Sep. 15(3):351-6. [QxMD MEDLINE Link].
Sakai S, Shida Y, Takahashi N, et al. Pulmonary lesions associated with visceral larva migrans due to Ascaris suum or Toxocara canis: imaging of six cases. AJR Am J Roentgenol. 186(6):1697-702. [QxMD MEDLINE Link].
Sane AC, Barber BA. Pulmonary nodules due to Toxocara canis infection in an immunocompetent adult. South Med J. 1997 Jan. 90(1):78-9. [QxMD MEDLINE Link].
Saporito L, Scarlata F, Colomba C, Infurnari L, Giordano S, Titone L. Human toxocariasis: a report of nine cases. Acta Paediatr. 2008 Sep. 97(9):1301-2. [QxMD MEDLINE Link].
Sharkey JA, McKay PS. Ocular toxocariasis in a patient with repeatedly negative ELISA titre to Toxocara canis. Br J Ophthalmol. 1993 Apr. 77(4):253-4. [QxMD MEDLINE Link].
Small KW, McCuen BW 2d, de Juan E Jr, Machemer R. Surgical management of retinal traction caused by toxocariasis. Am J Ophthalmol. 1989 Jul 15. 108(1):10-4. [QxMD MEDLINE Link].
Sturchler D, Schubarth P, Gualzata M, et al. Thiabendazole vs. albendazole in treatment of toxocariasis: a clinical trial. Ann Trop Med Parasitol. 1989 Oct. 83(5):473-8. [QxMD MEDLINE Link].
Szczepanski T, Sonta-Jakimczyk D, Janik-Moszant A, Olejnik I. Generalized lymphadenopathy as initial presentation of toxocariasis in a seven-year-old boy. Pediatr Infect Dis J. 1996 Aug. 15(8):717-8. [QxMD MEDLINE Link].
Taylor MR, Keane CT, O'Connor P, Mulvihill E, Holland C. The expanded spectrum of toxocaral disease. Lancet. 1988 Mar 26. 1(8587):692-5. [QxMD MEDLINE Link].
Taylor MR, O'Connor P, Hinson AR, Smith HV. Toxocara titres in maternal and cord blood. J Infect. 1996 May. 32(3):231-3. [QxMD MEDLINE Link].
Torgerson PR, Rosenheim K, Tanner I, Ziadinov I, Grimm F, Brunner M, et al. Echinococcosis, toxocarosis and toxoplasmosis screening in a rural community in eastern Kazakhstan. Trop Med Int Health. 2009 Mar. 14(3):341-8. [QxMD MEDLINE Link].
Uhlikova M, Hubner J. Seroprevalence of Toxocara canis infection in Czech Republic. Cent Eur J Public Health. 1998 Aug. 6(3):195-8. [QxMD MEDLINE Link].
Vidal JE, Sztajnbok J, Seguro AC. Eosinophilic meningoencephalitis due to Toxocara canis: case report and review of the literature. Am J Trop Med Hyg. 2003 Sep. 69(3):341-3. [QxMD MEDLINE Link].
Villano M, Cerillo A, Narciso N, et al. A rare case of Toxocara canis arachnoidea. J Neurosurg Sci. 1992 Jan-Mar. 36(1):67-9. [QxMD MEDLINE Link].
Walker MD, Zunt JR. Neuroparasitic infections: nematodes. Semin Neurol. 2005 Sep. 25(3):252-61. [QxMD MEDLINE Link].
Walsh SS, Robson WJ, Hart CA. Acute transient myositis due to Toxocara. Arch Dis Child. 1988 Sep. 63(9):1087-8. [QxMD MEDLINE Link].
Wells DL. Public understanding of toxocariasis. Public Health. 2007 Mar. 121(3):187-8. [QxMD MEDLINE Link].
Wisniewska-Ligier M, Wozniakowska-Gesicka T, Sobolewska-Dryjanska J, Markiewicz-Józwiak A, Wieczorek M. Analysis of the course and treatment of toxocariasis in children-a long-term observation. Parasitol Res. 2012 Jun. 110(6):2363-71. [QxMD MEDLINE Link]. [Full Text].
Wolfrom E, Chene G, Boisseau H, et al. Chronic urticaria and Toxocara canis. Lancet. 1995 Jan 21. 345(8943):196. [QxMD MEDLINE Link].
Xinou E, Lefkopoulos A, Gelagoti M, et al. CT and MR imaging findings in cerebral toxocaral disease. AJNR Am J Neuroradiol. 2003 Apr. 24(4):714-8. [QxMD MEDLINE Link].
Zachariah SB, Zachariah B, Varghese R. Neuroimaging studies of cerebral "visceral larva migrans" syndrome. J Neuroimaging. 1994 Jan. 4(1):39-40. [QxMD MEDLINE Link].
Zygulska-Mach H, Krukar-Baster K, Ziobrowski S. Ocular toxocariasis in children and youth. Doc Ophthalmol. 1993. 84(2):145-54. [QxMD MEDLINE Link].
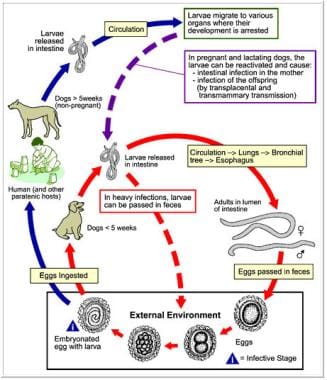 Diagram of the Toxocara canis life cycle image. Courtesy of the Centers for Disease Control and Prevention.
Diagram of the Toxocara canis life cycle image. Courtesy of the Centers for Disease Control and Prevention.
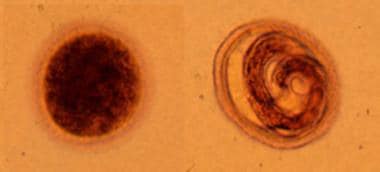 Toxocara canis eggs are passed in dog feces, especially puppies' feces. Humans do not produce or excrete eggs; therefore, the presence of these eggs is not a diagnostic finding in human toxocariasis. The egg to the left is fertilized but not yet embryonated, whereas the egg to the right contains a well-developed larva. The latter egg is infectious if it is ingested by a human (frequently, a child). Courtesy of the Centers for Disease Control and Prevention.
Toxocara canis eggs are passed in dog feces, especially puppies' feces. Humans do not produce or excrete eggs; therefore, the presence of these eggs is not a diagnostic finding in human toxocariasis. The egg to the left is fertilized but not yet embryonated, whereas the egg to the right contains a well-developed larva. The latter egg is infectious if it is ingested by a human (frequently, a child). Courtesy of the Centers for Disease Control and Prevention.





