Background
Supravalvar aortic stenosis (SVAS) is a fixed form of congenital left ventricular outflow tract (LVOT) obstruction that occurs as a localized or diffuse narrowing of the ascending aorta beyond the superior margin of the sinuses of Valsalva. [1] It accounts for less than 7% of all fixed forms of congenital LVOT obstructive lesions. SVAS is demonstrated in the images below. [2]
SVAS may occur sporadically, as a manifestation of elastin arteriopathy, or as part of Williams syndrome (also known as Williams-Beuren syndrome), an autosomal dominant genetic disorder. The sporadic form of SVAS is the most common (>50%) presentation. There is no known risk factor to account for these cases. (See Epidemiology and Etiology.)
A less common presentation of SVAS is a familial form caused by autosomal dominant inheritance. Like the sporadic form, it is not a part of Williams syndrome.
Patients with SVAS are usually asymptomatic, but cases associated with Williams syndrome are usually identified during infancy. The diagnosis of Williams syndrome can be established with cytogenic analysis, which means that this diagnosis can be made in utero using chorionic villus tissue. Therefore, SVAS can be detected prenatally, particularly in patients with Williams syndrome, if it is revealed with fetal echocardiography. (See Workup.)
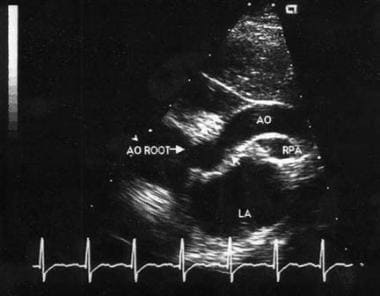
The anatomic diagnosis of SVAS can reliably be made from 2-dimensional (2D) echocardiography that uses multiple views, including parasternal, apical long-axis, and suprasternal (see Workup). Cardiac catheterization or MRI may be indicated to evaluate the coronary artery or aortic arch anatomy. Surgery is the primary treatment for SVAS. The choice of procedure varies with the type and severity of the stenosis. (See Treatment.)
Go to Aortic Stenosis for more complete information on this topic.
Anatomy
Supravalvar aortic stenosis (SVAS) has three commonly recognized morphologic forms. An external hourglass deformity of the aorta with a corresponding luminal narrowing at a level just distal to the coronary artery ostia is present in 50-75% of patients. [3] In approximately 25% of patients, a fibrous diaphragm is present just distal to the coronary artery ostia. In fewer than 25% of patients, a diffuse narrowing along a variable length of ascending aorta is present.
Similarly, the following three anatomic subtypes of coronary lesions have been recognized in SVAS [4] :
-
Circumferential narrowing of the left coronary ostium
-
Ostial obstruction due to fusion of the aortic cusp to the supravalvar ridge
-
Diffuse narrowing of the left coronary artery
Pathophysiology
The origins of the coronary arteries proximal to the obstruction site have the same systolic pressure as the left ventricle (LV), which is abnormally elevated based on the severity of obstruction. [3] Consequently, they become dilated and tortuous over time, with hypertrophy and intimal thickening. These changes predispose them to premature atherosclerosis. The hemodynamic consequences of coronary artery changes are increased total mean coronary flow but significantly decreased diastolic coronary flow, which is the major determinant of the development of myocardial ischemia.
Concentric LV hypertrophy caused by supravalvar aortic stenosis (SVAS) exacerbates the problem of myocardial ischemia. In most patients, the jet of blood flow from SVAS has a preferential trajectory into the brachiocephalic vessels, the so-called Coanda effect [5] ; this accounts for a marked increase in the right upper extremity systolic pressure relative to the left.
Complications of SVAS also include progressive coronary osteal stenosis, infective endocarditis, and sudden death. [6]
Etiology
The precise etiology of supravalvar aortic stenosis (SVAS) is unknown. The disease’s high association with Williams syndrome, a genetic disorder caused by a hemizygous deletion or mutation of the elastin gene at band 7q11, [7] suggests that defective connective tissue formation contributes to its pathology.
Patients with the sporadic form of SVAS may have associated peripheral pulmonary artery stenosis. There is no known risk factor for sporadic SVAS.
As previously stated, a less common presentation of SVAS is a familial form caused by autosomal dominant inheritance.
Epidemiology
The crude incidence of congenital heart defects is approximately 8 cases per 1000 live births. SVAS accounts for less than 0.05% of congenital heart defects. The sporadic form of SVAS is more common than the autosomal dominant form. [8]
As previously mentioned, the sporadic form of SVAS is the most common (>50%) presentation.
Prognosis
In one series, the actuarial survival rate following operative repair of supravalvar aortic stenosis (SVAS) was approximately 85% at 15 years. Overall survival, including operative mortality, was 98% at 10 years and 97% at 20 years and at 30 years. [9]
Postoperatively in this study, 73% of patients were in class I of the New York Heart Association's (NYHA) functional classification, and 27% were in NYHA functional class II. [9] Most patients did not require reoperation.
Prognosis is influenced by the presence of genetic disorders, coronary artery lesions, and associated obstructive lesions of pulmonary arteries. [10] SVAS is a progressive lesion, whereas peripheral pulmonary artery stenosis remains unchanged or decreases in severity over time. [11] The mortality rate is higher in patients with diffuse SVAS than in those with the localized form.
The risk of sudden cardiac death, including in patients who have undergone surgery, is 1 case per 1000 patient years and is 25-100 times higher than in the normal population. [12] Patients with SVAS are vulnerable to cardiac arrest or significant hemodynamic instability during induction of anesthesia secondary to hypotension and decreased coronary artery perfusion.
Anatomic abnormalities that predispose individuals with SVAS and Williams syndrome to sudden death include coronary artery stenosis and severe biventricular outflow tract obstruction. The mechanisms for sudden death for both anatomic subgroups are believed to include myocardial ischemia, decreased cardiac output, and arrhythmia. [13]
Patient Education
Preoperative recommendations for restriction of physical activities should be followed (see Activity). Physical activity restrictions are not required postoperatively if no residual lesion is present (including coronary artery involvement) and the pressure gradient is less than 20 mm Hg across the LVOT, which is similar to the preoperative recommendation.
In general, persons with SVAS should have risk stratification for coronary artery disease early in adult life, because SVAS may predispose the coronary artery to premature atherosclerotic changes.
-
Two-dimensional suprasternal echocardiographic image of supravalvar aortic stenosis.
-
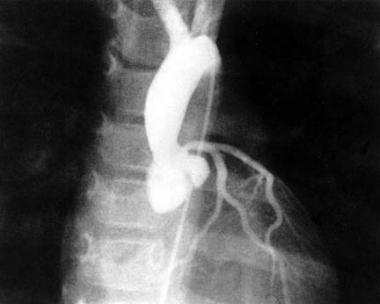
Aortogram of a patient with supravalvar aortic stenosis and dilated sinus of Valsalva.