Background
Childhood-onset schizophrenia is a severe form of psychotic disorder that occurs at age 12 years or younger and is often chronic and persistently debilitating, with worse outcomes than patients who have later onset of symptoms. [1] The definition of childhood schizophrenia has evolved over time and is now believed to be a virulent childhood version of the same disorder exhibited in adolescents and adults. The differentiation and significance of “childhood-onset,” versus “early onset,” versus “adult onset” is being explored, especially as it pertains to its utility to determine prognosis. One study of 88 patients found the age cut-off of 12 years old to lack utility. They instead suggested a cut-off age of 14.7 years of age based on higher levels of positive symptoms and poorer psychosocial functioning under this cut-off and better outcomes over this cut-off. [1]
The American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) cautions that although the essential features of schizophrenia are the same in childhood, it is harder to diagnose. Symptoms such as disorganized speech and behavior, which are typically present in schizophrenia, also occur in many disorders of childhood onset (e.g., autism spectrum disorder, obsessive compulsive disorder, [2] and attention-deficit hyperactivity disorder). It is important to consider these more common disorders of childhood before attributing symptoms to schizophrenia. [3]
Diagnostic criteria (DSM-5)
DSM-5 diagnostic criteria for schizophrenia requires at least two of the following five symptoms to be present for a month. [3] At least one of these must be (1), (2), or (3):
Delusions
Hallucinations
Disorganized speech
Grossly disorganized or catatonic behavior
Negative symptoms
Other criteria include a markedly lower level of functioning in one or more major areas, such as work or school, interpersonal relations or self-care; persistence of continuous signs of disturbance for at least 6 months; the ruling out of schizoaffective disorder; and the exclusion of substance abuse or another medical condition that may be causing the disturbance.
In patients with a history of autism spectrum disorder or a communication disorder of childhood onset, the additional diagnosis of schizophrenia is made only if prominent delusions or hallucinations, in addition to the other required symptoms or schizophrenia are also present for at least 1 month (or less if successfully treated).
In addition to the five symptom domain areas identified in the diagnostic criteria, the assessment of cognition, depression, and mania symptom domains is vital for making critically important distinctions between schizophrenia and other psychotic disorders. The American Psychiatric Association removed schizophrenia subtypes from the DSM-5 because they didn’t appear to help with providing better targeted treatment, or predicting treatment response. However, those individuals meeting the criteria for catatonia would receive an additional diagnosis of catatonia associated with schizophrenia to indicate the presence of the comorbidity.
The following duration specifiers are used only after 1-year duration of the disorder and they are not in contradiction to the diagnostic course criteria:
-
First episode, currently in acute episode
-
First episode, currently in partial remission
-
First episode, currently in full remission
-
Multiple episodes, currently in acute episode
-
Multiple episodes, currently in partial remission
-
Multiple episodes, currently in full remission
-
Continuous
The validity of a diagnosis of childhood-onset schizophrenia has been a point of concern for some, due to difficulty in differentiating pediatric patients’ reports of visual hallucinations from imaginary figures (which may be developmentally normal). One study on the validity of a diagnosis of early-onset schizophrenia in Denmark found a correspondence of 88.8%, comparing the diagnosis listed in the Denmark registry to a clinical diagnosis based on symptoms reported in patient records. The validity increased when the diagnosis was made on an inpatient unit (91.5%) as opposed to outpatient settings (71.9%). The validity of a diagnosis of the whole sample of early-onset schizophrenia was 83.5%, while the validity of only those with “very early onset schizophrenia” (synonymous with childhood-onset schizophrenia) was slightly lower at 82.8%. Interrater reliability was near-identical between the two groups. [4]
Etiology
No definite single etiology of schizophrenia has been identified. Most theories accept both genetic and environmental contributions for the causation of childhood-onset schizophrenia (COS).
A review of the data from the Environmental Risk Longitudinal Twin Study of British Children found that childhood psychotic symptoms are familial and heritable. These symptoms are associated with social risk factors; cognitive impairments at age 5 years; home-rearing risk factors; behavioral, emotional, and educational problems at age 5 years; and comorbid conditions such as self-harm. Therefore, childhood psychotic disorders may be a marker of an impaired developmental process. [5]
In addition, compared with the usual onset of schizophrenia in late adolescence or early adulthood, the emergence of earlier-onset schizophrenia during childhood may be due to increased genetic loading for schizophrenia or early central nervous system (CNS) damage due to an environmental factor.
Genetic risk
Several factors suggest a genetic risk. First-degree relatives of patients with early-onset schizophrenia (EOS) have a 5 to 20 times higher risk of developing schizophrenia compared to the general population. First-degree relatives of children with schizophrenia have a higher prevalence rate of schizophrenia and schizophrenia spectrum disorders. [6] A twin study in Denmark concluded the twin of a patient with schizophrenia had a 4.7 times increased risk of developing schizophrenia themselves if the first twin was diagnosed or presented with illness before 22 years old. They did not study more specific age ranges under 22 years old, but did say (based on their age divisions above 22 years) that overall decreasing age at first diagnosis significantly increased the risk of second-twin diagnosis. [7]
In the Pittsburgh High-Risk Study, findings among young relatives of schizophrenia patients included the following: [8]
-
High proportions of axis I psychopathology, especially attention deficit hyperactivity disorder (ADHD) and conduct disorder
-
Increased expressed emotion among relatives
-
A trend for more psychopathology in offspring of relatives with high expressed emotion
-
Impaired attention, spatial working memory, and executive functions
-
Increased soft neurologic signs
-
Volume reductions in the amygdala, hippocampus, and superior temporal gyrus
-
Decreased slow-wave sleep
First-degree relatives of individuals with schizophrenia have impairment in ocular smooth pursuit movements similar to that found on examination of patients with schizophrenia. One study found that healthy siblings of patients with COS had decreased cerebral gray matter in the same pattern as was seen in the patients. [9]
Examination of National Institute of Mental Health patients with onset of schizophrenia before age 13 years revealed a 10% rate of cytogenetic abnormalities. [10] Certain genome mutations have also been significantly linked with EOS including 1q21.1, 15q13.3, and 22q11.2 deletion syndrome and could account for 0.5%–1% of cases. In addition, associations with several schizophrenia-susceptibility genes in adult patient cohorts were replicated in the childhood-onset schizophrenia patients, including DAOA, NRG1, DTNBP1, and GAD1. [11] A study of Han Chinese patients found the gene rs139887 in SOX10 to be associated with males with early-onset schizophrenia. [12] A different study of 385 Han Chinese patients found a specific polymorphism in the serotonin 2A receptor to “confer susceptibility to schizophrenia with early age of onset.” [13] Rare genetic variations to miRNA (involved in brain development) were found to be 50% more prevalent in patients with early-onset schizophrenia than in the control population. [14] There was also an excess of novel copy number variants that overlapped or disrupted known genes in patients when compared with the nontransmitted parental “control” chromosomes. [15] A published case study in Boston revealed two patients who developed schizophrenia before 7 years of age who both possessed copy number variants at 16p13.11, a locus associated with both adult-onset schizophrenia and autism spectrum disorder. [16]
A study of 177 patients in Sweden found greater global DNA hypomethylation (conveying genetic instability) in leukocytes of patients with schizophrenia compared to controls, with this finding even more pronounced in patients with early-onset schizophrenia.{ref204-INVALID REFERENCE} Studies are underway to investigate the correlation of DNA methylation and gender differences in early-onset schizophrenia. [17]
Neurodevelopmental and neuroanatomical abnormalities
Several studies have described complications during pregnancy and delivery in adults who subsequently develop schizophrenia. The combination of genetic risk and evidence of acquired damage has suggested a neurodevelopmental theory with early CNS abnormalities that contribute to an increased vulnerability to schizophrenia later in life. An increase in minor dysmorphic features has suggested prenatal-onset problems. An increase in hypoxia-associated complications was demonstrated to increase the odds of developing earlier-onset schizophrenia.
The neuroanatomy of persons with COS has been examined by neuroimaging. As in adults with schizophrenia, the most consistent finding has been enlargement of the lateral ventricles. Although static in adults, the abnormalities in brain morphology evolve during adolescence. The possibility of a neurodegenerative process has been raised but also questioned. [18]
The literature has revealed a compelling story for gray matter deficits in individuals with COS. Specifically, Rapoport et al demonstrated that adolescents with schizophrenia have significantly greater decreases in frontal and temporal gray matter volumes than those observed in healthy age-matched controls (see the images below). [19, 20] The investigators additionally found the children with schizophrenia to have more cortical gray matter loss than children with transient psychosis.
Greenstein et al. reported that cortical thickness loss in childhood-onset schizophrenia appears to localize with age to prefrontal and temporal regions that are seen in patients with adult-onset schizophrenia, regardless of medication. [21] Another study showed that childhood-onset schizophrenia patients who met criteria for remission had thicker gray matter in prefrontal, temporal, and parietal cortices compared with nonremitted patients, suggesting a possible relation of brain plasticity with prognosis. [22]
The Edinburgh High-Risk Study suggested that in high-risk subjects (defined as subjects who had at least 2 close relatives with schizophrenia) the change from vulnerability to psychosis may be preceded by reduction in size and deteriorating function of the temporal lobe. [23]
In a systematic review and meta-analysis of 66 papers comparing brain volume in patients with a first psychotic episode with volume in healthy controls, meta-analysis suggested that the whole brain and hippocampal volume are reduced and that ventricular volume is increased in affected patients relative to healthy controls. [24] Furthermore, brain magnetic resonance scans obtained in childhood-onset schizophrenia subjects, their nonpsychotic full siblings, and matched health comparison subjects between the ages of 10 and 29 years that measured the total, left, and right hippocampal volumes showed that patients with childhood-onset schizophrenia had a fixed reduction in hippocampal volumes when compared with nonpsychotic siblings and health comparison subjects. In addition, no significant volumetric or trajectory differences were noted between nonpsychotic siblings and healthy comparison subjects. Therefore, decreased hippocampal volume may represent an intermediate disease phenotype. [25]
A study of siblings of patients with childhood-onset schizophrenia found an association between volumetric differences of the right superior frontal gyrus and cerebellum and late in learning performance on the weather prediction task (a test of cognitive skill learning). The authors suggested some of these volumetric abnormalities may be “potential endophenotype[s] for schizophrenia.” They suggested the genetic risk was “most apparent in adolescence” as these abnormalities normalized as the siblings reached adulthood. [26]
Studies of nonpsychotic siblings of childhood-onset schizophrenia patients have shown a pattern of prefrontal and temporal gray matter deficits during early ages that seem to normalize by the time the subjects reach late adolescence. [27] These results were replicated by Mattai et al., who also showed nonpsychotic siblings of childhood-onset schizophrenia patients have early gray matter deficits that improve over time, suggesting that late adolescence may be a critical time for greatest localization of deficits in probands or normalization in nonpsychotic siblings. [28]
A study looking at striatal volume and shape compared patients with childhood-onset schizophrenia, their siblings, and healthy controls. They found patients with childhood-onset schizophrenia displayed “subregional striatal shape differences,” particularly inward displacement of the anterior portion of the striatal head and outward displacement at the posterior portion of the striatal head. Siblings of these patients at least partially displayed these shape variations. [29] These findings are of particular interest as “tracts from the striatal head project extensively to the prefrontal cortex,” which shows cortical thickness deficits in patients with childhood-onset schizophrenia. [30]
Studies of white matter connectivity in childhood-onset schizophrenia have found abnormalities in the left and right cuneus (occipital lobe, visual cortex) in both patients with childhood-onset schizophrenia and their siblings, though they couldn’t find a statistically significant correlation between these abnormalities and the severity of clinical symptoms. [31] Another study of white matter abnormalities proposed abnormalities of the left inferior longitudinal fasciculus and left inferior fronto-occipital fasciculus as “possible biomarkers of vulnerability for developing schizophrenia.” [32] Another study observed white matter growth deficits in non-psychotic siblings of patients with childhood-onset schizophrenia, but these deficits normalized with age. [33]
A study reviewing MRI data asked if abnormal cortical maturation was confined to developmental modules in the brain. They found patients with childhood-onset schizophrenia had “altered maturational trajectories of cortical areas” involved in the cingulo-fronto-temporal developmental module. [34]
One study found patients with childhood-onset schizophrenia displayed “delayed maturation of occipitotemporal connectivity, with unaffected siblings displaying a milder phenotype.” This delay normalized in the unaffected siblings by mid-adolescence, and normalized in the patients with childhood-onset schizophrenia by early adulthood. Occipitotemporal connectivity is associated with the inferior longitudinal fasciculus, in which developmental delays may be associated with hallucinations. [35]
Patients diagnosed with childhood-onset schizophrenia were found to have reduced strength of short-distance functional connectivity, though “longer anatomical distances were relatively normal in the COS group.” The authors of that study found this to be consistent with the idea of “‘overpruning’ of short-distance connections” in childhood-onset schizophrenia. [36]
When studying working memory networks, it was found that patients diagnosed with early onset schizophrenia displayed a similar abnormal pattern of dorsolateral prefrontal cortex connectivity as adults diagnosed with schizophrenia. [37]
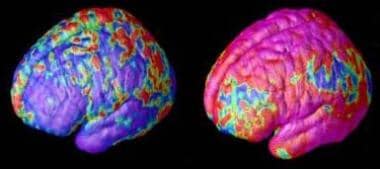 Childhood schizophrenia. Early and late gray matter deficits in schizophrenia. Areas of gray matter loss, shown in red and yellow, spread from back-to-front (right to left) over 5 years in composite MRI scan data from 12 teens with childhood-onset schizophrenia, beginning at age 14 (left). Red and yellow denotes areas of greater loss. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.
Childhood schizophrenia. Early and late gray matter deficits in schizophrenia. Areas of gray matter loss, shown in red and yellow, spread from back-to-front (right to left) over 5 years in composite MRI scan data from 12 teens with childhood-onset schizophrenia, beginning at age 14 (left). Red and yellow denotes areas of greater loss. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.
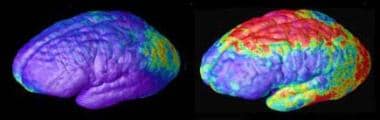 Childhood schizophrenia. Rate of gray matter loss. Composite MRI scan data showing areas of gray matter loss over 5 years, comparing 12 normal teens (left) and 12 teens with childhood-onset schizophrenia. Red and yellow denotes areas of greater loss. Front of brain is at left. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.
Childhood schizophrenia. Rate of gray matter loss. Composite MRI scan data showing areas of gray matter loss over 5 years, comparing 12 normal teens (left) and 12 teens with childhood-onset schizophrenia. Red and yellow denotes areas of greater loss. Front of brain is at left. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.
Cannabis use
Evidence from 6 longitudinal studies in 5 countries showed that regular cannabis use predicts an increased risk of a schizophrenia diagnosis or of reporting symptoms of psychosis. [38]
One study found that 74% of cannabis use disorders subjects had the onset of cannabis use disorder before the onset of positive psychotic symptoms. These subjects were predominately male, younger at study entry, had an earlier age at onset of positive symptoms, less educational attainment, a lower self-socioeconomic status, better premorbid childhood social adjustment, a trend for poorer premorbid childhood academic adjustment, less motor abnormalities, but more severe hallucinations and delusions.
However, in the multivariate analysis only male sex, worse socioeconomic status, better premorbid childhood social adjustment, and more severe positive symptoms at study entry were associated with a lifetime history of cannabis use disorder. The authors concluded that although cannabis use precedes the onset of illness in most patients, no significant association existed between onset of illness and cannabis use disorders that was not accounted for by demographic and clinical variables. [39]
Nevertheless, another study used the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS) to assess for a possible association between cannabis use, traumatic events, and psychotic symptoms in adolescence. Cannabis use and childhood trauma were significantly associated with a risk of experiencing psychotic symptoms. When cannabis use and childhood trauma occurred within the same patient, the risk for psychotic symptoms increased beyond the risk posed by either factor alone. However, only 14 participants in the study reported experiencing psychotic symptoms. Therefore, these findings must be replicated. [40]
A 2012 study obtained T1-weighted MRIs from adolescents with earlier-onset schizophrenia (EOS), cannabis use disorders (CUD), EOS plus CUD, and healthy controls. In the EOS group and the CUD group, the left superior parietal region had smaller gray matter volumes compared with healthy controls. The combined EOS/CUD group showed similar findings, but no additive effect was found. Nevertheless, the combined EOS/CUD group had smaller gray matter volumes in the left thalamus compared with the CUD and healthy control groups. [41]
Early adolescent cannabis use coupled with a specific genetic vulnerability may be a risk factor for the development of schizophrenia.
A 2015 literature review concluded that “cannabis alters the course of psychosis by triggering early onset of disease in a vulnerable youth population.” While multiple theories as to how this might happen were noted—substance abuse influencing psychiatric disorders, psychiatric disorders influencing substance abuse, independent and mutually exclusive processes—they refrained from ascribing to one particular mechanism. [42]
Early childhood trauma
As previously stated, early childhood trauma has been correlated with childhood psychotic symptoms. One study found 93.1% of patients with early-onset schizophrenia “had experienced adverse life events during childhood,” 46.9% had “experienced traumatic events.” [4]
Specifically, Arsenault et al. obtained data from the Environmental Risk longitudinal Twin Study, which interviewed mothers when their children were aged 5, 7, 10, and 12 years on whether the children had experienced maltreatment by an adult, bullying by peers, or involvement in an accident. When the children were aged 12 years, they were asked about bullying experiences and psychotic symptoms. Children who experienced maltreatment by an adult or bullying by peers were more likely to report psychotic symptoms at age 12 years than were children who did not experience maltreatment. The finding of higher risk of psychotic disorders was observed whether these events occurred early in life or later in childhood. In addition, this finding remained significant when sex, socioeconomic deprivation, internalizing or externalizing problems, children’s genetic liability to developing psychosis, and IQ were controlled. [43]
Furthermore a prospective cohort study of 12-year-old children assessed the risk of psychotic symptoms using the Psychosis-like Symptoms Interview. Children who had been victims of bullying at ages 8 and/or 10 years, independent of prior psychopathology, family adversity, or child’s IQ, had a 2-fold increase in risk of psychotic symptoms. The risk increased when victimization was chronic or severe. [44]
Infections
One study also found a link between viral CNS infections and later psychosis. Specifically, a national cohort of children born between 1973-1985 was followed by using Swedish national registers to determine the association between CNS infections at age 0-12 years and admissions for nonaffective psychotic illnesses from the 14th birthday (N=2269). There was a slightly increased risk of nonaffective psychotic illness associated with viral CNS infections, as well as schizophrenia. There was no increased risk in relation to bacterial infections. Exposure to mumps virus or cytomegalovirus were associated with later psychosis. [45] However further research into this correlation is needed.
Immunology
Some are focusing their attention on immunological markers as potential biomarkers of schizophrenia, some even working towards finding a causal relationship between immune processes and schizophrenia. One such study implicated an autoimmune component of schizophrenia, noting platelet-associated autoantibodies (PAA) to be elevated in patients with childhood-onset schizophrenia compared to a group of children diagnosed with conduct disorder. The authors of this study suggested measuring PAA as a possible diagnostic biomarker of childhood-onset schizophrenia. [46] Another study found a statistically significant correlation between negative symptoms of early-onset schizophrenia and IL-4 and IL-10 levels. [47]
Neurotransmitters and schizophrenia
Most psychologic, pharmacologic, and neuroimaging studies of childhood-onset schizophrenia have suggested dysfunction in the prefrontal cortex and limbic system. The neurotransmitter implicated in the pathophysiology of schizophrenia is dopamine. Medications that increase dopaminergic activity may induce a schizophreniform psychosis, and medications that block postsynaptic D2 receptors help alleviate symptoms of schizophrenia.
Other neurotransmitters may also be involved in the pathophysiology of schizophrenia. Glutamate has been implicated based, in part, on the production of psychotic symptoms by phencyclidine and the presence of N -methyl-D-aspartate (NMDA) receptor dysfunction. [48] Serotonin may be important. The new atypical antipsychotic medications have prominent serotonergic effects. Preliminary studies suggest gamma-aminobutyric acid (GABA) may be important. N-acetylaspartate may play a role as well, as one study found lower levels in the prefrontal cortex and thalamus in patients with early-onset schizophrenia. These levels responded and normalized after six months of treatment with atypical antipsychotics. [49]
Miscellaneous potential etiologies
No one single etiology has been identified for childhood-onset schizophrenia, and likely it is multifactorial. As such, many are attempting to identify potential causes, contributing factors, or biomarkers related to childhood-onset schizophrenia.
In a study of patients at a Nigerian tertiary care center, patients with childhood-onset versus adolescent-onset psychosis were more likely to have mothers who were ill during pregnancy or infancy. The authors suggested maternal illness may be a relevant component of childhood-onset schizophrenia. [50] Another study examined a possible link between early-onset schizophrenia and maternal small-cell lung cancer. Those authors suspected a connection due to the potentially prodromal aspect of the autoimmune nature of small-cell lung cancer. Their sample size was small, but they did find a statistically significant association between the two. [51]
Another study looked at the effect of oxidative stress in the pathogenesis of schizophrenia. The study authors found total antioxidant status was significantly associated with baseline cognitive function in early-onset psychosis (low total antioxidant status being associated with lower cognitive performance.) [52]
Epidemiology
Childhood-onset schizophrenia (COS) is rare in the United States; in preadolescents, the estimated prevalence is less than 1 case per 10,000 population. The number of new cases significantly increases during late adolescence, reaching an approximate prevalence of 1% for later-onset schizophrenia. Data from the British National Surveillance study suggests the 1-year incidence of COS of 0.21/100,000 and 1-year outcomes were poor. One study found that 11% of patients with First Episode Schizophrenia and 23% of patients at Clinical High Risk for psychosis reported remembering their first psychotic symptoms as presenting in childhood. [53]
One study in Denmark found the incidence of early-onset schizophrenia to increase when comparing the time period of 1971–1994 to 1994–2010. However, diagnostic criteria for early-onset schizophrenia changed during this time including letting go of a previous restriction that only allowed the diagnosis to be made in inpatient settings. While diagnostic incidence may have increased, it may have been accounted for by these changes in diagnostic criteria. [54]
Globally, schizophrenia with an onset later in life appears to have an equal prevalence, with a possible increase in prevalence in urban populations. Most studies demonstrate an average male-to-female incidence of schizophrenia of 1.5–2:1; this ratio is continually being re-examined and challenged. [54] Males have a slight but statistically significant earlier age at onset, as well as higher rates of comorbid pervasive developmental disorder (PDD) and attention deficit/hyperactivity disorder (ADHS) when compared to females. The higher rates of comorbid PDD and ADHD with childhood-onset schizophrenia, however, mirror the same male-to-female ratios found in adult-onset schizophrenia. [55]
No studies of overall prevalence of childhood-onset schizophrenia in underdeveloped countries are available. A study of 409 pediatric patients with psychotic symptoms in Nigeria found 40.8% to have a diagnosis of schizophrenia, with 8.1% of the 409 patients being younger than 12 years old. Compared to pediatric patients with adolescent-onset psychotic symptoms, patients with childhood-onset psychotic symptoms were found to have less family history of psychiatric illness, more maternal illness during pregnancy, more illness in infancy, and overall more diagnoses of psychotic disorder due to another general medical condition as opposed to other diagnoses of psychosis. [50]
The 2006 Aetiology and Ethnicity in Schizophrenia and Other Psychoses Study (AESOP), a large, population-based case-control study conducted over two years in three study centers in England in adults, reported all psychoses were more common in the black and minority ethnic group compared with the white British group. [53] A British study of early-onset non-affective psychosis published in 2015 found no “preference” of gender, race, or ethnicity. [56]
One Finnish study also of early-onset schizophrenia found a statistical significance regarding birth interval. Patients with early-onset schizophrenia more often were born within 1–2 years of their nearest sibling. [57] Interestingly, the effect of season of birth on diagnosis of schizophrenia has been the focus of multiple studies. A 2017 study of data from Korean patients was consistent with studies of other nationalities finding a predominance of winter births (particularly January and February) for patients diagnosed with early-onset schizophrenia. [58]
In a child younger than 13 years, the onset of schizophrenia is rare and is generally insidious, carrying a worse prognosis. Onset of the disorder in the adolescent years is more common and may have an acute or insidious onset. In general, the earlier the onset of schizophrenia, the poorer the outcome.
Prognosis
The prognosis for childhood-onset schizophrenia and adolescent-onset schizophrenia is worse than that observed in adult-onset schizophrenia. As adults, these children experience the following:
-
Fewer close social relationships
-
Less likely to be married [59]
-
Less academic achievement
-
More unemployment
-
Less capacity for independent living
Patients with an onset before adolescence and those with an insidious onset appear to have a worse response to medication and a worse prognosis. Patients with early-onset non-affective psychosis displayed multiple readmissions and trials of multiple antipsychotics at one-year follow up. [56]
An increased risk of death from suicide is present in patients with schizophrenia. In large follow-up studies of childhood-onset schizophrenia, the mortality rate from suicide is 5–11%. Approximately 10% of adults with schizophrenia commit suicide. Children and adolescents with psychotic disorders were more likely to display suicidal behaviors (ideation 32% vs 6.7%, planning 20% vs 0%, attempts 20% vs 0%), and more likely to display these behaviors than even children and adolescents diagnosed with depression. No difference was appreciated in rates of suicidal behavior between children versus adolescents with a psychotic disorder. [60]
Violence is also a potential problem, particularly for the adolescent with paranoid ideation. Patients diagnosed with early-onset schizophrenia spectrum disorder were more likely to display violence before 15 years old, and “present early conduct problems,” but were also less likely to be violent after 18 years old compared to patients diagnosed with adult-onset schizophrenia. [59] Other complications arise from poor self-care, impulsivity leading to injury or sexually acquired diseases, and substance abuse. One study found early-onset psychosis (not just schizophrenia) was associated with more agitation and aggression, lifetime substance use disorder, antisocial personality disorder, and interaction with the legal system. [61]
In follow-up studies, more than 50% of children with schizophrenia have persistent severe impairment in social skills and limitations in academic and occupational achievement. One study published in 2016 found patients with childhood-onset schizophrenia spectrum disorder were twice as likely to be diagnosed with ADHD and twice as likely to have speech, language, and learning disabilities compared to patients with adolescence-onset schizophrenia. [62] Patients with early-onset schizophrenia performed worse on neurocognitive tests compared to the control group. But even more interestingly, patients with early-onset schizophrenia and a family burden of psychosis performed worse than those without a family burden of psychosis. [63]
The duration of untreated psychosis has been a point of study for many as it appears to have implications in prognosis, especially at two years. A longer duration of untreated psychosis along with a more severe clinical picture at first presentation was associated with a poorer two-year course. The authors of that study speculated this association to be a finding of arrested development rather than deterioration. [64] A longer duration of untreated psychosis was also associated with lower rates of PANSS remission at two-year follow-up. [65] Conversely, a shorter duration of untreated psychosis was associated with greater improvements in executive function. [66]
One small study found shorter gestational length did not increase the risk of early-onset schizophrenia diagnosis, but it did worsen neurocognition within the early-onset schizophrenia population. [67]
Patient Education
Psychoeducation is essential for families of children with schizophrenia. They need to be educated about the causes, symptoms, natural history, therapy, adverse effects of medication, and complications of childhood-onset schizophrenia.
Families must also know the warning signs of impending relapse. High levels of expressed emotion have been associated with an increased risk of relapse in adults with schizophrenia and can possibly contribute to problems in children with schizophrenia.
Once children with schizophrenia are in remission, teach them to self-monitor for signs of possible relapse. Inform these children about possible adverse effects of medication.
For patient education information, see Mental Health and Behavior Center, as well as Schizophrenia.
-
Childhood schizophrenia. Early and late gray matter deficits in schizophrenia. Areas of gray matter loss, shown in red and yellow, spread from back-to-front (right to left) over 5 years in composite MRI scan data from 12 teens with childhood-onset schizophrenia, beginning at age 14 (left). Red and yellow denotes areas of greater loss. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.
-
Childhood schizophrenia. Rate of gray matter loss. Composite MRI scan data showing areas of gray matter loss over 5 years, comparing 12 normal teens (left) and 12 teens with childhood-onset schizophrenia. Red and yellow denotes areas of greater loss. Front of brain is at left. Source: Paul Thompson, MD, UCLA, Laboratory of Neuroimaging. NIMH media file.







