Practice Essentials
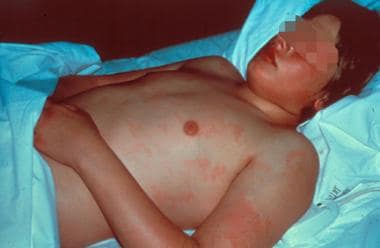
Rheumatic fever (RF) is a systemic illness that may occur following group A beta hemolytic streptococcal (GABHS) pharyngitis in children. Rheumatic fever and its most serious complication, rheumatic heart disease (RHD), are believed to result from an autoimmune response; however, the exact pathogenesis remains unclear. Erythema marginatum, the characteristic rash of acute rheumatic fever, is shown below.
Background
Studies in the 1950s during an epidemic on a military base demonstrated 3% incidence of rheumatic fever in adults with streptococcal pharyngitis not treated with antibiotics. [1] Studies in children during the same period demonstrated an incidence of only 0.3%. The incidence of rheumatic fever after GABHS infection is thought to be decreased to less than 1%. Cardiac involvement is reported to occur in 30-70% of patients with their first attack of rheumatic fever and in 73-90% of patients when all attacks are counted.
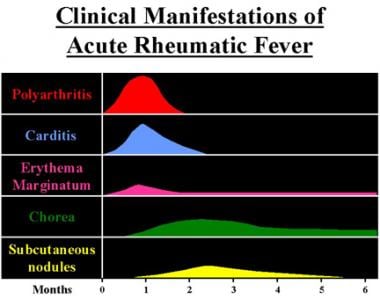
Clinical manifestations and time course of acute rheumatic fever are shown in the image below.
Pathophysiology
Rheumatic fever develops in children and adolescents following pharyngitis with GABHS (ie, Streptococcus pyogenes). The organisms attach to the epithelial cells of the upper respiratory tract and produce a battery of enzymes, which allows them to damage and invade human tissues. After an incubation period of 2-4 days, the invading organisms elicit an acute inflammatory response, with 3-5 days of sore throat, fever, malaise, headache, and elevated leukocyte count. In a small percent of patients, infection leads to rheumatic fever several weeks after the sore throat has resolved. Only infections of the pharynx have been shown to initiate or reactivate rheumatic fever. However, epidemiological associations in certain populations have led to speculation that group A Streptococcus impetigo could predispose to or cause rheumatic fever as well. [1]
Direct contact with oral (PO) or respiratory secretions transmits the organism, and crowding enhances transmission. Patients remain infected for weeks after symptomatic resolution of pharyngitis and may serve as a reservoir for infecting others. Penicillin treatment shortens the clinical course of streptococcal pharyngitis and more importantly prevents the major sequelae.
GABHS organisms are gram-positive cocci, which frequently colonize the skin and oropharynx. These organisms may cause suppurative diseases (eg, pharyngitis, impetigo, cellulitis, myositis, pneumonia, puerperal sepsis). GABHS organisms also may be associated with nonsuppurative diseases (eg, rheumatic fever, acute poststreptococcal glomerulonephritis). Group A streptococci (GAS) elaborate the cytolytic toxins, streptolysins S and O. Of these 2 toxins, streptolysin O induces persistently high antibody titers that provide a useful marker of GAS infection and its nonsuppurative complications. Recent studies using enzyme-linked immunosorbent assays showed a correlation between anti-streptolysin O and anti-human cardiac myosin antibodies. [2]
GAS, as identified using the Lancefield classification, has a group A carbohydrate antigen in the cell wall that is composed of a branched polymer of L-rhamnose and N-acetyl-D-glucosamine in a 2:1 ratio. Surface proteins on the cell wall of the organism may subserotype GAS. The presence of the M protein is the most important virulence factor for GAS infection in humans. More than 120 M protein serotypes or M protein genotypes have been identified, [3] some of which have a long terminal antigenic domain (ie, epitopes) similar to antigens in various components of the human heart.
Rheumatogenic strains are often encapsulated mucoid strains, rich in M proteins, and resistant to phagocytosis. These strains are strongly immunogenic, and anti-M antibodies against the streptococcal infection may cross-react with components of heart tissue (ie, sarcolemmal membranes, valve glycoproteins). Currently, emm typing is felt to be more discriminating than M typing. [3]
Acute RHD often produces a pancarditis, characterized by endocarditis, myocarditis, and pericarditis. Endocarditis is manifested as mitral and aortic valve insufficiency. Severe scarring of the valves develops during a period of months to years after an episode of acute rheumatic fever, and recurrent episodes may cause progressive damage to the valves. The mitral valve is affected most commonly and severely (65-70% of patients); the aortic valve is affected second most commonly (25%).
The tricuspid valve is deformed in only 10% of patients, almost always in association with mitral and aortic lesions, and the pulmonary valve is rarely affected. Severe valve insufficiency during the acute phase may result in congestive heart failure (CHF) and even death (1% of patients). Whether myocardial dysfunction during acute rheumatic fever is primarily related to myocarditis or is secondary to CHF from severe valve insufficiency is not known. When pericarditis is present, it rarely affects cardiac function or results in constrictive pericarditis.
Chronic manifestations occur in adults with previous RHD from residual and progressive valve deformity. RHD is responsible for 99% of mitral valve stenosis in adults, and it may be associated with atrial fibrillation from chronic mitral valve disease and atrial enlargement
Etiology
Rheumatic fever is believed to result from an autoimmune response; however, the exact pathogenesis remains unclear.
Rheumatic fever only develops in children and adolescents following group A beta hemolytic streptococcal (GABHS) pharyngitis, and only infections of the pharynx initiate or reactivate rheumatic fever.
At least some rheumatogenic strains of GAS have antigenic domains similar to antigens in components of the human heart, and some authors have proposed that anti-M antibodies against the streptococci may cross-react with heart tissue, causing the pancarditis that is observed in rheumatic fever. So-called molecular mimicry between streptococcal and human proteins is felt to involve both the B and T cells of peripheral blood, with infiltration of the heart by T cells. Some believe that an increased production of inflammatory cytokines is the final mechanism of the autoimmune reaction that causes damage to cardiac tissue in RHD. An insufficiency of interleukin-4 (IL-4)–producing cells in the valve tissue may also contribute to the valve lesions.
Streptococcal antigens, which are structurally similar to those in the heart, include hyaluronate in the bacterial capsule, cell wall polysaccharides (similar to glycoproteins in heart valves), and membrane antigens that share epitopes with the sarcolemma and smooth muscle.
Decreased levels of regulatory T-cells have also been associated with rheumatic heart disease and with increased severity. [4]
In utero precursors predisposing to rheumatic heart disease have also been proposed; Eriksson et al suggest increased spiraling of the umbilical cord may increase the risk of developing rheumatic heart disease secondary to presumed changes in hemodynamic conditions during formation of the mitral valve. [5]
Epidemiology
United States statistics
Rheumatic fever is now uncommon among children in the United States. The incidence of rheumatic fever and RHD was thought to be decreased in the United States and other industrialized countries during the past 80 years. The prevalence of RHD in the United States was less than 0.05 per 1000 population, with only rare regional outbreaks reported in Tennessee in the 1960s and in Utah, [6] Ohio, and Pennsylvania in the 1980s. In the early 1900s, the incidence was reportedly 5-10 cases per 1000 population.
Decreased incidence of rheumatic fever was attributed to the introduction of penicillin or a change in the virulence of the streptococci, whereas the incidence in other developed countries, such as Italy, was comparable. [7] However, an assessment of temporal trends of patients diagnosed with acute rheumatic fever in the United States from 2001-2011 showed that since 2001, national acute rheumatic fever admissions have steadily increased, with a peak in 2005, and decreased thereafter. [8] The annual incidence of acute rheumatic fever in the United States is fewer than 2 cases per 100,000 school‐aged children. [9]
International statistics
In contrast to trends in the United States, rheumatic fever and RHD have not decreased in developing countries. The incidence of acute rheumatic fever throughout the world is 8-51 per 100,000 people, and children aged 5-15 years are most commonly affected. [10] Retrospective studies in developing countries demonstrate the highest figures for cardiac involvement and the highest recurrence rates of rheumatic fever. [11] Worldwide, there are over 15 million cases of RHD, with 282,000 new cases and 33,000 deaths from this disease each year. [12]
Race-, sex-, and age-related demographics
Race
Native Hawaiians and Maori (both of Polynesian descent) have a higher incidence of rheumatic fever. Incidence of rheumatic fever in these patients is 13.4 per 100,000 hospitalized children per year, even with antibiotic prophylaxis of streptococcal pharyngitis. [12]
The prevalence is greatest in the South (34.32%) compared to the Northeast (25.05%), Midwest (22.95%), and West (17.69%). Blacks have the highest mortality rate (5.00%) compared to Whites (3.01%), Hispanics (1.66%) and Asians (0.89%). As median income decreases, the frequency increases, with patients earning less than $25,000 per year making up 23.74% of admissions. [8]
Sex
Rheumatic fever occurs in equal numbers in males and females. Females with rheumatic fever fare worse than males and have a slightly higher incidence of chorea.
Age
Rheumatic fever is principally a disease of childhood, with a median age of 10 years. However, GABHS pharyngitis is uncommon in children younger than 3 years, and acute rheumatic fever is extremely rare in these younger children in industrialized countries. Although less commonly seen in adults compared with children, rheumatic fever in adults accounts for 20% of cases.
Prognosis
The manifestations of acute rheumatic fever resolve during a period of 12 weeks in 80% of patients and may extend as long as 15 weeks in the remaining 20% of patients.
Rheumatic fever was the leading cause of death in patients aged 5-20 years in the United States 100 years ago. At that time, the mortality rate was 8-30% from carditis and valvulitis but decreased to a 1-year mortality rate of 4% by the 1930s. Following the development of antibiotics, the mortality rate decreased to nearly 0% by the 1960s in the United States. However, the mortality rate has remained 1-10% in developing countries.
The development of penicillin also has affected the likelihood of developing chronic valvular disease after an episode of acute rheumatic fever. Prior to penicillin, 60-70% of patients developed valve disease; since the introduction of penicillin, 9-39% of patients develop valve disease.
In patients who developed murmurs from valve insufficiency from acute rheumatic fever, numerous factors (eg, severity of initial carditis, presence or absence of recurrences, amount of time since episode of rheumatic fever) affected the likelihood that valve abnormalities and the murmur would disappear. The type of treatment and the promptness of its initiation did not affect the likelihood that the murmur would disappear. In general, incidence of residual rheumatic heart disease (RHD) at 10 years was 34% in patients without recurrences but was 60% in patients with recurrent rheumatic fever. In patients in whom the murmur disappeared, it did so within 5 years in 50%. Thus, a significant number of patients experience resolution of valve abnormalities even 5-10 years after their episode of rheumatic fever.
The importance of preventing recurrences of rheumatic fever is evident.
Morbidity/mortality
RHD is the major cause of morbidity from rheumatic fever and is the major cause of mitral insufficiency and stenosis in the United States and the world. Variables that correlate with severity of valve disease include the number of previous attacks of rheumatic fever, the length of time between the onset of disease and start of therapy, and sex (the prognosis for females is worse than for males). Insufficiency from acute rheumatic valve disease resolves in 70-80% of patients if they adhere to antibiotic prophylaxis.
Complications
Potential complications include CHF from valve insufficiency (acute rheumatic fever) or stenosis (chronic rheumatic fever).
Associated cardiac complications include atrial arrhythmias, pulmonary edema, recurrent pulmonary emboli, infective endocarditis, thrombus formation, and systemic emboli.
Patient Education
Emphasize measures that minimize further damage to the valves of the heart.
Timely evaluation and treatment of pharyngitis in children help prevent rheumatic fever.
Secondary prophylaxis of patients with previous rheumatic fever and valve involvement with penicillin injections every 3-4 weeks decrease the recurrence of RHD.
Additional prophylactic antibiotics prior to dental and surgical procedures decrease the likelihood of bacterial endocarditis.
-
Clinical manifestations and time course of acute rheumatic fever.
-
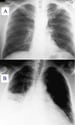
Chest radiograph showing cardiomegaly due to carditis of acute rheumatic fever.
-
Erythema marginatum, the characteristic rash of acute rheumatic fever.