Background
Retinopathy of prematurity (ROP) is a serious vasoproliferative disorder that affects the retina of extremely premature infants. Milder forms of ROP often regress or heal without intervention; however, more advanced stages can lead to severe visual impairment or blindness. ROP remains a serious problem despite striking advances in neonatology: it can lead to lifelong disabilities for the smallest survivors of neonatal intensive care units (NICUs).
Pathophysiology
Retinopathy of prematurity (ROP) primarily occurs in extremely low birth weight (ELBW) infants. Most research suggests that a low birth weight, a young gestational age (GA) (see the Gestational Age from Estimated Date of Delivery calculator), and the severity of illness (eg, respiratory distress syndrome [RDS], bronchopulmonary dysplasia [BPD], sepsis) are associated risk factors. Other associations have been described; however, the severity of systemic illness appears to be a major predictor of severe disease. The smallest, sickest, and most immature infants are at the highest risk for serious disease. Race is also a factor: Black infants appear to have less severe ROP. [1, 2]
In essence, an early stage of retinal microvascular degeneration is followed by neovascularization that has the potential for the development of retinal detachment and permanent loss of vision. [3] A review by Fevereiro-Martins et al indicates that key contributory factors during this early stage include oxidative and nitrosative stress, as well as inflammatory processes. Nitric oxide synthase and arginase contribute to ischemia/reperfusion-induced neurovascular degeneration, mediators of the hypoxia-inducible factor pathway (eg, vascular endothelial growth factor [VEGF], metabolic factors such as succinate) drive destructive neovascularization, and the extracellular matrix is involved in hypoxia-induced retinal revascularization. Revascularization of the avascular zone is prevented by vasorepulsive molecules (semaphorin 3A). [3]
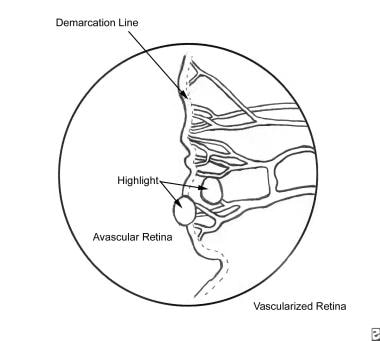
Retinal vasculature begins to develop around 16 weeks' gestation. It extends from the optic nerve head centrifugally toward the periphery. Full vascular maturation of the retina typically occurs near term (40 weeks). Premature birth results in a disruption of normal retinal vascular maturation. Exposure of newborn premature infants to hyperoxia downregulates retinal VEGF. Blood vessels constrict and can become obliterated, resulting in delays of normal retinal vascular development. This hyperoxia-vasocessation results in avascular peripheral retina, and it is seen clinically as stage 0 or stage 1 of retinopathy of prematurity. See the image below.
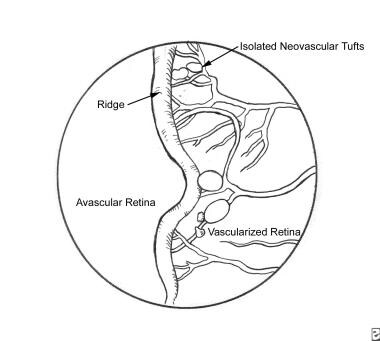
Early on, oxygen and nutrients can be delivered to the retina by means of diffusion from the rich vascular bed beneath the retina known as the choroid. The retina continues to grow in thickness and eventually outgrows this vascular supply, and the inner retina must receive oxygen and nutrients from the retinal vessels. Prolonged retinal hypoxia leads to an upregulation of VEGF, and the growth of abnormal/extraretinal vessels. Stage 2 ROP represents the first appearance of this abnormal growth, and it is seen clinically as a ridge at the border of the vascular-avascular retina (see the image below).
In addition to VEGF, this process is mediated by insulinlike growth factor-1 (IGF-1) and other cytokines.
Further growth of the abnormal vasculature tends to occur out of the plane of the retina and into the vitreous (stage 3 ROP). This is known clinically as neovascularization, because the vessels are largely incompetent, leaking proteins and other cytokines into the vitreous where they can precipitate localized contraction of the gel. This contraction can lead to traction and, eventually, elevation (detachment) of the retina (stages 4 and 5 ROP).
"Plus disease" is the dilation and tortuosity of the normal retinal vessels in the posterior pole (most posterior part) of the retina.
Dhaliwal et al found that ROP occurred with significantly greater frequency and severity in small-for-GA (SGA) infants compared with appropriate-for-GA (AGA) infants. [4] In a review of 1413 infants with birth weight less than 1500 g and/or GA of 26-31 weeks, infants with a birth weight below the tenth percentile for GA were more likely to develop any stage of ROP than their AGA peers (P< 0.01), and they were more likely to develop severe ROP (GA of 26-27 weeks, P< 0.01; GA of 28-31 weeks, P = 0.01).
Epidemiology
United States data
The incidence of retinopathy of prematurity (ROP) varies with birth weight, but it is reported to be approximately 50-70% in infants whose weight is less than 1250 g at birth.
in a retrospective review (1989-1997), Hussain et al analyzed the incidence and the need for surgery in neonates with ROP who were born at 22-36 weeks' gestation. [5] The incidences were 21.3% (202 of 950 patients) for ROP of any stage and 4.6% (44 of 950 patients) for ROP at stage III or worse. No ROP was noted in infants born after 32 weeks' gestation, and no infant born after 28 weeks' gestation needed retinal surgery in this study. Despite the increased survival of extremely low birth weight (ELBW) infants, they found a considerable reduction in the incidence and severity of ROP compared with reports from an earlier period. [5] However, infants born before 28 weeks' gestation and those with birth weights less than 1000 g were at risk to need retinal surgical treatment for ROP.
Investigators from the Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP) multicenter trial concluded that maintaining oxygen saturation in the high-90% range did not reduce the severity of the retinopathy when compared with the saturations in the low-90% range. [6] However, it did result in more adverse pulmonary events. In a subanalysis of infants who did not have plus disease (ie, tortuosity of vessels) at the time of study entry, the progression to threshold was significantly decreased when compared with the progression in infants with plus disease. [6] Thus, a critical window for oxygen administration may be determined.
International data
ROP is prevalent worldwide, and several reports have detailed the incidence and risk factors associated with the disease.
A Korean study reported a 20.7% incidence of ROP (88 of 425 premature babies), with a gestational age of 28 weeks or less and a birth weight of 1000 g or less were the most significant risk factors. [7] A study from Singapore reported a 29.2% incidence of ROP (165 of 564 ELBW infants), with a median age of onset of 35 weeks (range, 31-40 wk) postmenstrual age. [8] The risk factors for development of threshold ROP by regression analysis were maternal preeclampsia, birth weight, pulmonary hemorrhage, duration of ventilation, and duration of continuous positive airway pressure (CPAP). [8]
An observational study from United Kingdom designed to compare the characteristics of infants with severe ROP in countries with low, moderate, and high levels of development found that the mean birth weights of affected infants from highly developed countries was 737-763 g compared with 903-1527 g in less-developed countries. [9] Mean gestational ages of affected infants from highly developed countries were 25.3-25.6 weeks compared with 26.3-33.5 weeks in less-developed countries. Thus, larger and more mature infants seemed to be developing severe ROP in less-developed nations. This suggests that individual countries need to develop their own screening programs with criteria suited to their local population.
Race-, sex-, and age-related demographics
Some reports indicate a decreased incidence of progression to threshold disease in Black infants. Most evidence comes from the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) study. [10] Further evidence that Black infants are less likely to develop severe ROP has been reported in studies of candidemia in ELBW infants. [11] The exact mechanism for the decreased incidence of progression to surgery in Black infants has not been described. Bizzaro et al showed a strong genetic predisposition to ROP when comparing monozygotic twins with dizygotic twins. [12]
Although some reports indicate a male predilection, the CRYO-ROP study revealed no differences based on sex. [10]
ROP is a disease of the immature retina, and the occurrence of ROP is inversely related to gestational age. Generally, infants born at 32 weeks or greater have an extremely low risk of ROP. EBLW infants, especially those with a more unstable clinical course (more comorbidities), are more likely to develop and require treatment for ROP.
Prognosis
The prognosis of retinopathy of prematurity (ROP) is predicted by the disease stage.
Patients who did not progress beyond stage 1 or stage 2 have a good prognosis, as do most successfully treated babies with zone II/III disease.
Patients with posterior zone I disease or stage 4 have a guarded prognosis for their vision. Infants with stage 5 disease mostly have extremely poor vision.
Long-term outcomes for serious disease, especially those babies who received treatment for ROP, include severe visual impairment and blindness. Infants with advanced ROP may develop vision-threatening conditions such as myopia, amblyopia, and strabismus, and these infants require close follow-up after discharge from the neonatal intensive care unit. Repka et al described the need for subsequent ophthalmic intervention in patients with ROP. [13]
The structure of the retina, especially the macula, of infants with ROP does not closely correlate with the function (vision). Specialized testing (Teller Acuity Cards) may be necessary early on to fully appreciate the visual function of each eye. Significant differences in the acuity between the eyes can rapidly lead to amblyopia of the poorer-seeing eye.
Complications
Late complications of ROP include myopia, amblyopia, strabismus, nystagmus, cataracts, retinal breaks, and retinal detachment.
VanderVeen et al observed strabismus is often variable and may improve by age 9 months. [14]
Follow-up by an ophthalmologist is required on a long-term basis (see "Long Term Monitoring" in the Treatment section).
The peripheral retina of adults with regressed or treated ROP is not normal. The abnormal development of the peripheral retina can lead to thinning (lattice degeneration) of the peripheral retina, retinal holes, tears, and detachments later in life. [15] Young adults with treated or regressed ROP should be counselled about these risks and should have regular dilated funduscopic examinations by a retina specialist. Special attention should be paid to teenagers who engage in contact sports; they and their parents should be taught the signs and symptoms of retinal tears and detachments. More frequent examinations (eg, at the start and conclusion of their sport's season) may be prudent.
-
Retinopathy of Prematurity. Stage I retinopathy of prematurity.
-
Retinopathy of Prematurity. Scheme of retina of right eye (RE) and left eye (LE) showing zone borders and clock hours used to describe location and extent of retinopathy of prematurity.
-
Retinopathy of Prematurity. Zone I retinopathy of prematurity.
-
Retinopathy of Prematurity. Zone II retinopathy of prematurity.
-
Retinopathy of Prematurity. Zone III retinopathy of prematurity.
-
Retinopathy of Prematurity. Stage II retinopathy of prematurity.
-
Retinopathy of Prematurity. Stage III retinopathy of prematurity.
-
Retinopathy of Prematurity. Mild extraretinal fibrovascular proliferation.
-
Retinopathy of Prematurity. Moderate extraretinal fibrovascular proliferation.
-
Retinopathy of Prematurity. Severe extraretinal fibrovascular proliferation.
-
Retinopathy of Prematurity. A comparison between attached and detached retina.
-
Retinopathy of Prematurity. Retinopathy of prematurity (ROP) threshold, according to the Cryotherapy for Retinopathy of Prematurity (CRYO-ROP) Cooperative Group.
-
Retinopathy of Prematurity. Treatment guidelines, according to the Early Treatment for Retinopathy of Prematurity (ET-ROP) study.
-
Retinopathy of Prematurity. Laser photocoagulation. RPE = retinal pigment epithelium.
-
Retinopathy of Prematurity. Cryotherapy probe application.