Practice Essentials
Rabies is caused by a neurotropic virus of the family Rhabdoviridae, genus Lyssavirus, subgroup rabies virus. [1]
Globally, rabies is designated a Neglected Tropical Disease by the World Health Organization and accounts for over US$8 billion in annual economic costs. Children of poor and rural areas are predominantly affected, [2] and Africa and Asia shoulder 95% of associated deaths. The average cost of rabies postexposure prophylaxis (about US$100) puts lifesaving treatment tragically out of reach for much of the world. [3]
The virus commonly is transmitted via saliva that contaminates bites, scratches, and wounds, and, recently, via mucosal exposure. [4, 5] Rabies transmission via transplanted neurologic tissues (corneas) and solid organs also has been documented. [6, 7, 8] One case of rabies was reported in China after exposure of an open wound to the blood of a person bitten by a dog; the exposed person succumbed to rabies after seeking no medical care, while the bitten individual received postexposure prophylaxis and did not develop rabies. [9]
Animal species that present the highest transmission risk to humans include canines (domestic dogs predominantly, as well as foxes and coyotes), cats, raccoons, skunks, and bats. In many areas where canine rabies has been well-controlled and declared free of canine rabies, sylvatic rabies remains an endemic source in wildlife. [5] Rabies variants have been detected in cougars and skunks in Mexico, which was declared free of human rabies from dogs in 2021. Interestingly, the lowest-risk species is the opossum, in which the virus does not replicate because of its low body temperature.
In the United States, bats now account for over 90% of unrecognized, untreated exposures since 1990, resulting in one to two preventable deaths annually. [10] Owing to the subtlety of exposures (saliva aerosols or saliva contaminating tiny bites or mucous membranes), bats may pose the highest and least-recognized risk by the general public. Vaccine hesitancy poses a challenge as well. One of three deaths attributed to bats in 2021 occurred after refusal of post-exposure prophylaxis, due to "long-standing fear of vaccines." [10] Exposure may go unrecognized by a sleeping individual; thus, postexposure prophylaxis (PEP) is recommended whenever a bat is discovered in the room of a sleeping or incapacitated person.
A bat appearing docile on the ground is ill and presumably rabid. Bats being small, furry, and potentially irresistible to curious humans, children should be taught not to handle a downed bat. An adult might do so with great care and thick protective gloves or a container, but only to put the animal out of the reach of curious pets and people. Rabies avoidance and capture recommendations may be found at the Centers for Disease Control and Prevention (CDC).
Human-to-human rabies virus transmission via saliva theoretically is possible. Although it has not been documented, a mucosal, scratch, or bite exposure to saliva or blood of a person (or any other mammal) suspected of having rabies would be managed in a manner similar to that of any other exposure, with rabies PEP.
Standard precautions are recommended in the care of patients with rabies in healthcare settings, including use of personal protective equipment during activities that may pose a risk for salivary contamination of mucosa or break in the skin. [11]
Rabies PEP should begin as soon as possible aftter an exposure. PEP is safe and nearly 100% effective if administered before symptom onset. Depending on site and exposure, rabies may incubate for many years, and a subtle exposure may be forgotten. [12] Although the incubation period of rabies is typically 1-3 months, this may be shortened to a few days if inoculation occurs on the head and neck. Given that rabies invariably is fatal once symptoms begin, elapsed time should not discourage prophylaxis, and it is never too late to prophylax a possible rabies exposure. [5]
(See Presentation.)
Background
The fatal madness of rabies has been described throughout recorded history, and its association with rabid canines is well known. For centuries, dog bites were treated prophylactically with cautery, with predictable and unfortunate results. In the 19th century, Pasteur developed a vaccine that successfully prevented rabies after inoculation and launched a new era of hope in the management of this uniformly fatal disease. (See Treatment and Medications.)
Rabies is a viral disease that affects the central nervous system (CNS). The genus Lyssavirus contains more than 80 viruses. Classic rabies, the focus of this article, is the prototypical human Lyssavirus pathogen. [1] (See Etiology.)
There are 10 viruses in the rabies serogroup, most of which only rarely cause human disease. The genus Lyssavirus, rabies serogroup, includes the classic rabies virus, Mokola virus, Duvenhage virus, Obodhiang virus, Kotonkan virus, Rochambeau virus, European bat Lyssavirus types 1 and 2, and Australian bat Lyssavirus. [1] (See Etiology.) Five antigenic variants of rabies strains are recognized in the United States.
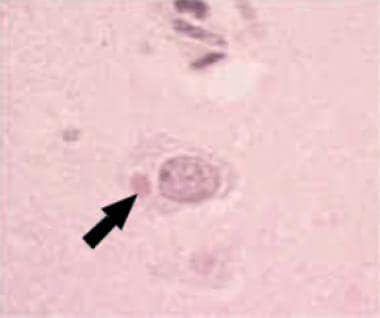
The rabies virus is a bullet-shaped virion with a single-stranded ribonucleic acid (RNA) nucleocapsid core and lipoprotein envelope. Its nucleocapsid material consists of Negri bodies, which are observed in the cytoplasm of infected neurons. The virus is transmitted in saliva or in aerosolized secretions from infected animals, typically via a bite. The virus is not hardy and is quickly inactivated by drying, ultraviolet rays, x-rays, trypsin, detergents, and ether.(See Etiology.)
Etiology
Rabies is a highly neurotropic virus that evades immune surveillance by its sequestration in the nervous system. Upon inoculation, it enters the peripheral nerves. A prolonged incubation follows, the length of which depends on the size of the inoculum and its proximity to the CNS. Amplification occurs until bare nucleocapsids spill into the myoneural junction and enter motor and sensory axons. At this point, prophylactic therapy becomes futile, and rabies can be expected to follow its fatal course, with a mortality rate of 100%.
The rabies virus travels along these axons at a rate of 12-24 mm/d to enter the spinal ganglion. Its multiplication in the ganglion is heralded by the onset of pain or paresthesia at the site of the inoculum, which is the first clinical symptom and a hallmark finding. From here, the rabies virus spreads quickly, at a rate of 200-400 mm (7-15 inches) per day, into the CNS, and spread is marked by rapidly progressive encephalitis. Thereafter, the virus spreads to the periphery and salivary glands, where it may be transmitted to others.
From the standpoint of diagnosis and therapeutic opportunities, it is important to understand that rabies does not damage neurons. Neuronal morphology and lifespan is normal throughout the course of the disease. Death occurs from global neurotransmitter blockade and widespread neurologic dysfunction. The virion acts in the synaptic space, where homology in amino acid sequences between neurotransmitter receptors for acetylcholine, GABA, and glycine may afford a mechanism for viral binding of these receptors. Thus, its action is neurotoxic, rather than cytotoxic.
Ironically, as disease progresses, virus may no longer be viable or replicating in tissue, although Negri bodies are present.
If the virus could be contained or the binding action reversed, complete recovery indeed might be possible.
Epidemiology
United States
Rabies is recognized as a global zoonosis yet remains remarkably neglected, despite unmatched lethality. It remains a threat underappreciated by healthcare practitioners in many endemic areas, often owing to lack of rapid diagnostic tools, postmortem evaluation, and public health reporting. Further, few resources have been devoted to its mechanisms of disease and potential therapeutic targets; the therapeutic approach remains a crude guess at best, based on anecdotal experiences shared across the globe. Most attention has focused on preventive strategies, which are fortunately highly effective where implemented.
The prevalence of rabies varies by location depending on animal-control effectiveness and immunization programs. The largest number of human deaths annually was recorded during the first half of the 20th century, with an average of 50 documented cases per year. Most were related to rabid-dog exposure. After 1940, when canine rabies vaccination programs began, the average number of documented cases declined to 2 per year. From 2001-2005, 15 cases of human rabies were reported in the United States.
Human rabies reflects the prevalence of animal infection and the extent of contact this population has with humans. Less than 5% of cases in developed nations occur in domesticated dogs; however, unvaccinated dogs serve as the main reservoir worldwide. Undomesticated canines, such as coyotes, wolves, jackals, and foxes, are most prone to rabies and serve as reservoirs. [5] These reservoirs allow rabies to remain an indefinite public health concern, and ongoing public health measures are critical to its control. Animal-control and vaccination strategies currently supersede postexposure prophylaxis in preventing the spread of rabies. Through such programs, rabies has been eliminated in some parts of the United States and several nations.
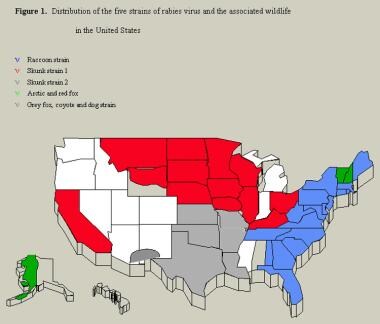
In the United States, rabies is most common in raccoons on the eastern coast and in skunks, foxes, coyotes, and dogs on the Texas-Mexico border. Bats, however, account for the highest number of rabies deaths in the United States, because these exposures are more likely to go unrecognized [5] ; inoculation with infected saliva may occur via tiny bites or scratches, mucosal contact (eyes, mouth), or theoretically, aerosolized saliva. Canine rabies, and to a lesser extent, bat rabies are significant problems in Mexico and around the world. (Opossums arerarely infected and are not considered a likely risk for exposure.)
The only rodent in the United States that can carry rabies long enough to transmit it to humans is the groundhog. Other small rodents (eg, squirrels, chipmunks, rats, mice) and lagomorphs (eg, rabbits, hares) usually die before being able to transmit rabies virus to humans, and human disease has not been documented from these mammals.
Domestic animals usually succumb to the virus strain predominant in their geographic region. Other cases have been associated with dog or animal bites in travelers returning from abroad, especially in countries where wild canine rabies is endemic. In other countries, canines are the most common source of rabies. Other animals, such as mongooses, jackals, ferrets, and domestic farm animals, may be common sources. Human-to-human transmission has only occurred with corneal and other organ transplants. [6, 7] Transmission of virus in saliva through mucous membranes, open wounds, or scratches is possible but rarely documented.
Rabies continues to adapt to new hosts and evolve transmissibility in previously “dead-end” hosts. In Arizona 2001, a mutated bat strain was confirmed to have developed both pathogenicity and transmissibility in both foxes and skunks, which previously were not seriously affected or contagious upon infection. Human encroachments into natural areas, as in suburban development, have been associated with the spread of rabies strains in the past. [13]
In addition, changes in epidemiology are expected to follow global climate change and are most likely to be detected in areas of climate extremes. This is being illustrated in Alaska, as increased viral transmission shifts from red fox to arctic fox populations following warming trends. Increased surveillance is needed to improve predictive models of epidemiology and human risk. [14]
Bats
Bat (avian) rabies appears to be widespread in the 49 continental states, and since 1980, most endemic rabies cases in humans in the United States have been associated with bat strains. [15]
Bat bites, if noticed by the patient, generally are thought to be trivial injuries because of the small size of most temperate-zone species (eg, silver-haired bats, eastern pipistrelles). In addition, bat bites can go completely unrecognized by the patient; consequently, appropriate postexposure prophylaxis is not administered.
One third of rabies cases occur in children, and most have no known exposure to a rabid animal. Because children may not be able to recall contact with a bat, if a bat is found in a room where a child has been sleeping, the bat should be captured and submitted for examination to the county or state health authorities. In 60% of cases, testing of the bat can avoid the need for rabies immunization. [16]
At least 30 of the more than 39 species of bats in the United States have been reported as rabid at some time.
Raccoons
Raccoons have been a recognized a reservoir for rabies in the southeastern United States since the 1950s. [17] The risk for raccoon transmission exists in all of the eastern coastal states and Alabama, Pennsylvania, Vermont, West Virginia, and Ohio.
Skunks
Three areas are associated with skunk-borne rabies: the north-central United States, the south-central United States, and California. As recently as 2001, a new skunk-borne variant arose from a bat strain and since has been quickly spreading.
Dogs and cats
Cats are the most common domestic animals reported by US health departments as being rabid, owing to unvaccinated strays that have possible contacts with bats and other mammals. [18, 19]
Dogs and cats along the Mexican border
Limited resources and minimal public health infrastructure in the bordering communities have hindered efforts to maintain animal control through dog-vaccination programs. Viral studies of human cases reported from US border states implicate an urban canine rabies strain and a link to coyote rabies in southern Texas. [20]
Lower-risk animal species in the United States
Any mammal is potentially at risk for rabies, some more than others. Lower-risk animal species in the United States include dogs, cats, and ferrets in areas not near a border. No person in the United States has ever contracted rabies from a dog, cat, or ferret held in quarantine for 10 days.
American opossums are especially at low risk, because the species’ low body temperature hinders replication.
Animal rabies vaccine
The vaccinia-rabies glycoprotein virus used in rabies vaccine–laden baits for wild animals is a self-replicating agent. This oral animal vaccine may cause adverse effects in some humans exposed to it through animal bites, particularly in hosts with altered immunocompetence and persons in whom smallpox vaccination is contraindicated (eg, pregnant women, patients with an exfoliative skin condition). [21, 22]
Transplantation patients
The innate state of immunosuppression in this population often provides a favorable environment for viral replication. Recipients of neurally derived tissues are at highest risk; however, any tissue poses a risk. In 2004, kidneys and liver were inadvertently transplanted from a donor from Texas with undiagnosed rabies; the recipients developed clinical rabies within 30 days, resulting in 100% mortality. [23]
International
Rabies is more prevalent in the developing world than in industrialized countries. The World Health Organization (WHO) estimates that rabies is responsible for 35,000 to 50,000 deaths annually worldwide and that gross underreporting is likely. An estimated 10 million people receive postexposure prophylaxis each year after being exposed to animals with suspected rabies. Unvaccinated dogs are the major reservoir for rabies.
Global reservoirs of rabies virus are as follows [24, 25] :
-
Europe - Foxes, bats
-
Middle East - Wolves, dogs
-
Asia - Dogs
-
Africa - Dogs, mongooses, antelopes
-
North America - Foxes, skunks, raccoons, insectivorous bats
-
South America - Dogs, vampire bats
Gender or sex-related demographics
Encounters with rabid animal vectors may be increased in males, who may have greater contact in certain geographic areas. Evidence to support this is found in data on dog bites, which are observed more frequently in males than in females.
Pathophysiology
Rabies virus infection is remarkable for the lack of evident pathology despite dramatic neurologic symptoms. Minimal inflammation and neuronal cytopathy may be observed even postmortem. Similarly, viremia does not occur or play a role in spread to the CNS.
Pathophysiology has been best characterized in canine rabies variants. Canine rabies in humans requires deep-muscle inoculation. Endogenous muscle micro-RNA bind to viral transcripts and limit both replication and viral protein production, such that the virus is able to evade detection by antigen-presenting cells. Once enough virus replicates (or with a high-level inoculum or direct nerve injury), it binds motor neuron junctions at postsynaptic nicotinic acetylcholine receptors, which initiates uptake into the motor endplate. From here, the virus rapidly propagates across motor axons and chemical synapses in retrograde fashion toward the ganglia and nerve roots, at which point the prodromal symptoms of neuralgia and hypoesthesia may begin, in addition to fever and flulike illness.
Once reaching the CNS, it spreads throughout via the more ubiquitous nicotinic acetylcholine receptors of the brain. Of note, anterograde spread of rabies virus may then occur via sensory and autonomic pathways from the CNS to viscera, explaining many of the symptoms of progressive disease. Throughout propagation of the virus along motor pathways, the virus elicits little inflammation, and the motor neurons continue their otherwise normal functions of neurotransmission. Increasing signs of inflammation develop as CNS and visceral spread occurs, although a significant paucity of findings remains, other than mild nonspecific MRI T2 enhancements. Spinal fluid remains largely acellular, even in the presence of detectable rabies virus.
Propagation to the CNS via peripheral sensory or autonomic synapses does not seem to occur with canine variants, and only about 30% of cases result in peripheral sensory neuralgia. However, 70% of cases with bat variants result in neuropathic pain in the region of inoculation, as well as Horner syndrome and other findings; thus, these alternate pathways may occur in bat rabies variants. [26, 27]
Prognosis
Despite aggressive and intensive care management, the prognosis of rabies remains exceedingly poor. The very few cases of survival have involved bat variants.
Patient Education
The general public should be aware of the risk for rabies with wild and feral domestic canine breeds, cats, raccoons, and, in particular, bats, which often appear to be innocuous or produce small injuries that may be dismissed. Bats found in a room with a sleeping child or incapacitated individual should prompt rabies prophylaxis. Animals with rabies may act unusually docile or be found out and about in daylight when otherwise nocturnal, or the animal may be unusually aggressive. Thus, the concept of "unprovoked" versus "provoked" injury is not useful in determining rabies risk. [5] Children should be taught not to pet or handle wild animals. A bat on the ground or easily caught should be considered rabid.
Prompt washing of potentially rabies-exposed wounds or mucosa with soap and water is important and may significantly reduce viral inoculum.
Travelers to areas endemic for rabies and who may participate in activities that may increase the exposure risk (eg, exploring caves or ruins, exposure to wild or feral animals) should seek rabies preexposure prophylaxis or be aware of sites to access reliable postexposure prophylaxis in the area. CDC Travel offers comprehensive guidance based on type of travel, region, and activity for the public and healthcare professionals.
-
Hematoxylin and eosin stain of Negri body in a rabies-infected neuron. Courtesy of the US Centers for Disease Control and Prevention.
-
Distribution of the 5 strains of rabies virus and the associated wildlife in the United States.