Overview
Streptococcus pneumoniae, or pneumococcus, is an encapsulated Gram-positive bacterium that is the major cause of bacteremia and upper respiratory infections (eg, otitis media and sinusitis) in children and a common cause of serious invasive infections. Because pneumococcus commonly and asymptomatically colonizes the upper respiratory tract of children, a breakdown of the normal mucosal barrier is believed to play a major role in the development of bacteremia. [1]
Occult bacteremia is defined as the presence of bacteria in the bloodstream of a febrile child who has no apparent focus of infection [2] and clinically does not appear to be ill. Some experts include in this definition children who have otitis media at their initial presentation and are subsequently found to have positive blood cultures.
Pneumococcal bacteremia was termed “pneumococcal fever” during the 1970s and 1980s, when S pneumoniae was recovered in approximately 5% of blood cultures from young febrile children with mild upper respiratory tract changes (see the table below). [3] Such changes included inflammation of the tympanic membranes, pharyngeal erythema, sinus clouding, and interstitial markings on chest roentgenograms.
Table 1. Incidence of Bacteremia in Febrile Children (Open Table in a new window)
Study |
Incidence |
McGowan (1973) [4] |
4% (outpatients) |
Teele (1975) [5] |
3.2% |
McCarthy (1977) [6] |
7.3% |
Waskerwitz (1981) [7] |
5.8% |
Dershewitz (1983) [8] |
4.3% |
Carroll (1983) [9] |
10.4% |
Bennish (1984) [10] |
4.3% |
Jaffe (1987) [11] |
2.8% |
Lee (1998) [12] |
1.6% |
In studies conducted before 1990, S pneumoniae,Haemophilus influenzae type b (Hib) and Neisseria meningitidis were recovered from approximately 75%, 20%, and 5%, respectively, of positive blood cultures in patients with occult bacteremia, with occasional isolates of Salmonella and Staphylococcus aureus. [4, 5, 13, 14, 15]
Subsequently, Salmonella bacteremia has been shown to occur in children with Salmonella gastroenteritis much more commonly than was previously thought. This organism is now the second most common pathogen in children with identified bacteremia. [12]
Severe infections, predominantly meningitis, occur in fewer than 6% of pneumococcal occult bacteremia cases—a figure that is much lower than the 50% reported with meningococcal bacteremia and the 20% reported with H influenza. [16]
Occult bacteremia now occurs in only 1 of 200 children who present with acute fever (temperature of 39o C [102.2o F] or higher) and white blood cell counts of 15,000/µL or higher. The most likely cause of bacteremia remains S pneumoniae; when there is no evidence of toxicity, such bacteremia is generally a benign, self-limited event.
Because of the extremely low yield, blood cultures are no longer routinely warranted in children aged 3-36 months who have no obvious source of infection, and empiric treatment of occult bacteremia is no longer appropriate. Almost all cases will spontaneously resolve with a low rate of subsequent focal infection. If a child remains febrile and worsens clinically, further diagnostic evaluation and possible empiric treatment with antibiotics pending results of cultures may be considered.
The identification of patients at risk for bacteremia and the formulation of strategies to prevent secondary complications (eg, meningitis, pneumonia, septic arthritis, osteomyelitis, and cellulitis) remain important aspects of general pediatric practice.
For patients with focal infection and pneumococcal bacteremia, treatment of the focal infection and monitoring for improvement are standard. For outpatients with proven pneumococcal bacteremia, reevaluation of their clinical status and identification of any new focus of infection is essential.
Epidemiology
Occult pneumococcal bacteremia is most common in children aged 3-36 months, an age at which children no longer have maternal IgG but have not yet developed their own protective antibodies. Before the availability of conjugate pneumococcal vaccine, 3-5% of children aged 3-36 months who had a fever higher than 39°C and no source of infection were bacteremic. In the fully immunized child, the incidence is now estimated to be much lower, approximately 0.5%.
In the United States, shortly before the introduction of the heptavalent conjugate pneumococcal vaccine (PNCV7) , the incidence of invasive pneumococcal infection, primarily bacteremia, was 23.2 cases per 100,000 population, including 3,000 cases of meningitis and 50,000 cases of bacteremia per year. [17, 18] Pneumococcus is still the most common cause of bacterial meningitis and bacterial pneumonia in US children younger than 2 years.
A study by Greenhow et al that retrospectively reviewed 57,733 blood cultures from children 3 to 36 months old reported that the incidence of Streptococcus pneumoniae bacteremia decreased from 74.5 per 100,000 children to 3.5 per 100,000 in the post-PCV-13 period, a 95.3% reduction. [19]
Clinical Presentation and Laboratory Features
The most likely cause of bacteremia remains S pneumoniae. In the absence of apparent toxicity, pneumococcal bacteremia is a benign, transient, and self-limited event. [20, 21, 12, 22, 8] It should be differentiated both from bacteremia involving more virulent organisms and from sepsis, in which blood cultures are repeatedly positive and the patient exhibits signs and symptoms of severe illness. [23, 24] Repeated positive cultures also suggest the development of a focal infection. [23, 24, 25]
Earlier studies of pneumococcal bacteremia in children identified a significantly higher incidence of infection in patients aged 7-12 months with temperatures of 39.4°C to 40.6°C (103°F to 105°F) and white blood cell (WBC) counts higher than 20,000/µL. [5] Another study reported a 3-fold greater incidence of bacteremia when the WBC count was over 15,000/µL and the erythrocyte sedimentation rate (ESR) exceeded 30 mm/hr. [6]
Other studies, however, varied in their support of and conclusions about whether the WBC is a useful indicator for bacterial disease. [7] In one report, only 6.9% of patients with a WBC count higher than 15,000/µL were found to have positive blood cultures, whereas 35% of the bacteremic patients had counts below 15,000/µL. [4, 11]
Febrile seizures have been a presenting symptom in as many as 67% of patients with pneumococcal bacteremia. [2, 3, 4, 5, 26] This association is difficult to interpret, in that both febrile seizures and pneumococcal bacteremia are independently most common in infants and children between 6 and 36 months of age.
Occult bacteremia is also more frequent in febrile children with an initial diagnosis of an upper respiratory tract infection/fever of unknown origin or pneumonia [22] than in those with otitis media or pharyngitis. Invasion of the respiratory passes by pneumococcus apparently predisposes to bacteremia. [1]
Among the factors that have not been found to have a significant effect on the incidence of occult bacteremia are sickle cell disease, [27, 28] hemoglobin sickle cell disease, and sickle cell trait. [29] Most reports have not shown a gender-, race-, or socioeconomic-related predominance of the disease. [2]
History and Physical Examination
History
In young patients with high fever, a careful history and physical examination are needed to identify possible sources of infection. In children with positive blood cultures, the duration of fever has usually been brief. Approximately 40% of patients with pneumococcal bacteremia have had fever for less than 1 day, and 82% of patients have had fever for less than 2 days. [2, 3, 4, 5]
Elements of the history that have been associated with an increased risk of pneumococcal bacteremia include daycare attendance, lack of breastfeeding, and underlying illness (eg, asplenia, immunodeficiency, or AIDS). [30] Although recent antibiotic use does not affect the overall rate of infection, children who were treated with antibiotics in the preceding 30 days are more likely to be infected with S pneumoniae that is resistant to penicillin. [30]
A longitudinal study of invasive pneumococcal infections revealed that a history of an underlying medical condition was a significant risk factor for increased mortality. Children with invasive pneumococcal infections and underlying comorbidity had a mortality of 3.4%, whereas previously healthy children with invasive pneumococcal infections had a mortality of 0.84%. [31, 30]
Physical examination
By definition, occult pneumococcal bacteremia occurs in children who are febrile and may have some degree of irritability or lethargy but do not show signs of focal infection and do not have a toxic appearance. Patients with recognizable viral illnesses (eg, stomatitis, croup, bronchiolitis, varicella, and mononucleosis) are at lower risk for pneumococcal bacteremia.
Potential underlying causes of pneumococcal bacteremia that should be apparent on physical examination include the following:
-
Meningitis
-
Sepsis
-
Cellulitis
-
Osteomyelitis
-
Septic arthritis
-
Pneumonia
Laboratory Studies
The need for laboratory tests should be determined on the basis of the patient’s history (including his or her functional status [ie, toxic vs nontoxic appearance]) and the physical examination results. No single laboratory test has yet shown sufficient sensitivity and specificity to adequately direct patient management in cases of acute fever without an apparent focus. The single exception is a screening urinalysis or urine culture, which is recommended for all febrile infants and young children who have no focus of infection. [32]
Bennish et al found that the combined quantitative C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR) had better sensitivity and specificity for occult bacteremia than the total white blood cell (WBC) count, the polymorphonuclear (PMN) cell count, the band cell count, or the patient’s temperature. [10] The sensitivity of all these tests, with the exception of temperature elevation, was increased when fever had been present for more than 24 hours.
Blood culture
Because of the extremely low yield, blood cultures are no longer routinely warranted in children aged 3-36 months who have no obvious source of infection, and empiric treatment of occult bacteremia is no longer appropriate. In a fully immunized febrile child, the calculated likelihood of a positive blood culture is less than 1 in 200 and 94% of all cases of pneumococcal bacteremia spontaneously resolve, with a low rate of subsequent focal infection. If a child remains febrile and worsens clinically, further diagnostic evaluation and possible empiric treatment with antibiotics pending results of cultures may be considered.
A blood culture should be considered in patients aged 3-36 months who are thought to be at risk for bacteremia as determined by ill appearance, focal infection, or high fever. Occult pneumococcal bacteremia may yield fewer than 10 colony-forming units (CFU)/mL, a figure that is lower than that seen in focal disease.
Chest Radiography
The only imaging study to be considered in infants and children with fever without a source is chest radiography to evaluate for pneumonia. Specific physical examination findings supporting this study include tachypnea, grunting, flaring, retracting, rhonchi, wheezing, rales, and focal decreased breath sounds, which are 94-99% specific for pneumonia.
Among these findings, tachypnea is the most common. [33] Evaluation for pneumonia in febrile children who do not manifest any of these findings is of very low yield.
Empiric Antibiotic Therapy for Suspected Occult Bacteremia
Current consensus on empiric treatment
Empiric antibiotic therapy for children with high fever and no identifiable focus of infection has always been highly controversial. The current consensus, however, is that empiric treatment of occult bacteremia is no longer appropriate.
Ceftriaxone, a third-generation cephalosporin with an extended half-life, has provided the potential for managing many serious pediatric infections with a single daily IM injection on an outpatient basis. General dosing recommendations for ceftriaxone are 50-75 mg/kg/day, in 1 or 2 doses. The serum concentrations obtained with IM administration of ceftriaxone are comparable to those obtained with IV administration, and the drug is generally well tolerated, except for occasional diarrhea and rare anaphylactic reactions.
The primary concern with bacteremia is that serious infections, such as meningitis, may develop in some infected children. The best documentation of these more serious consequences of pneumococcal bacteremia was published in the 1970s; in these reports, 6% of cases of pneumococcal bacteremia resulted in severe infection. [16, 9]
In weighing the current value of empiric antimicrobial therapy for the outpatient management of fever, although oral or parenteral therapy may improve the clinical appearance of children with bacteremia without decreasing the development of major focal infections, it does not benefit the much larger number of children who are not bacteremic.
We can now calculate the current risk of severe infection in patients who present with fever and no focus of infection. According to data collected between 1993 and 1996, [12] most cases of bacteremia (92%) are pneumococcal; however, the incidence of pneumococcal bacteremia is greatly reduced (70-90%) among children who have been immunized. [34] . Therefore, more than 3000 blood cultures would have to be obtained to pick up a single case of bacteremia that might result in severe infection.
At this rate, empiric testing and treatment would certainly not be cost-effective. This conclusion reflects current experience throughout the country [35] and emphasizes the need to reexamine our conventional wisdom as it pertains to the management of febrile children. Accordingly, most experts no longer recommend empiric therapy for low-risk children or for those who do not appear toxic (see the table below).
Table 2. Suggested Management of Patients With Suspected Occult Bacteremia (Open Table in a new window)
Evaluation |
Complete history and physical examination Complete blood cell count with differential Blood culture Urinalysis with culture Chest roentgenogram (optional) Consider throat culture (older child) Consider lumbar puncture (younger child) |
Treatment options |
Acetaminophen for fever control and observation at home for symptoms of worsening condition Follow-up appointment if indicated by child’s appearance Empiric antibiotics only for high-risk children |
Evolving evidence against cost-effectiveness of empiric treatment
A prospective study by Jaffe et al, which found no difference in the incidence of major infectious morbidity for occult pneumococcal bacteremia in patients receiving either oral amoxicillin or placebo, argues against empiric treatment—except for those children considered at high risk on the basis of age (< 28 days) or toxicity. [11] Antimicrobial therapy produced a more rapid defervescence in the bacteremic patients, but it did not alleviate symptoms in those who were not bacteremic.
Subsequently, a meta-analysis of 10 evaluable oral antibiotic treatment studies noted only a modest decrease in the risk of serious bacterial infections in children with S pneumoniae occult bacteremia. [36] The authors concluded that there was insufficient evidence that oral antibiotics prevent severe infections, including meningitis.
Of the 2 studies that employed parenteral ceftriaxone, [20, 21] one concluded that treatment was effective in preventing H influenzae meningitis but had no effect on the long-term outcome in patients with pneumococcal bacteremia. [21] Bass and colleagues also found that febrile children who were treated with ceftriaxone had a greater reduction in fever and improvement in their mean clinical scores, regardless of whether they were bacteremic or not. [20]
Clinical investigations conducted in the 1980s [11, 9] and the early 1990s [20, 21] on febrile high-risk children demonstrated that antimicrobial treatment—either oral or parenteral—improved the outcome of children with suspected bacteremia.
A consensus paper published in Pediatrics (the official journal of the American Academy of Pediatrics) in July 1993 concluded that “children 3 to 36 months of age with fever greater than 39°C and whose WBC count is 15,000/µL or more should have a blood culture and be treated with antibiotics pending culture results.” [37] This practice guideline was extremely influential among pediatricians in their management of infants and children with fever.
Data from the 1990s (including a study conducted from 1993 to 1996 on febrile children 3-36 months old) indicated that the only organism commonly responsible for bacteremia in the post– Hinfluenzae type b (Hib) era is pneumococcus, accounting for 92% of isolates, with Salmonella accounting for only 5% and group A beta-hemolytic streptococci only 1%. [12]
However, of almost 10,000 blood cultures obtained in this study, only 1.6% were positive. The authors emphasized that the total absence of Hib as a cause of bacteremia was a consequence of universal vaccination against this pathogen. [12]
A meta-analysis by Baraff and coworkers that included all published data on early institution of either oral or parenteral antibiotics likewise concluded that intramuscular (IM) or intravenous (IV) ceftriaxone had only a modest impact on the incidence of H influenzae disease. [38]
Subsequent studies examined the impact of the heptavalent pneumococcal conjugate vaccine (PNCV7) on pneumococcal bacteremia and invasive disease. [34, 39] The introduction of this vaccine led to a 70-90% reduction in bacteremia and pneumococcal invasive disease. [40, 41, 42, 43]
There is also evidence of a herd effect, a modest reduction of disease caused by nonvaccine strains, and a decrease in antibiotic resistance among strains causing disease. [34] By 2001, the overall rate of invasive pneumococcal disease in children younger than 2 years had declined by 78%. [39]
A study involving Medicaid patients in Tennessee, which focused on medical visits for pneumonia or invasive pneumococcal disease, determined that emergency department visits for these indications had decreased by 18% and outpatient visits by 17%. [44] A similar review of occult pneumococcal bacteremia in New York determined that outpatient visits had declined by 34%. [35]
Experience to date would now confirm that among children who have received both Hib and PNCV7 vaccines and who subsequently have an episode of high fever and leukocytosis, the rate of bacteremia would be lower than 0.5%.
The real question today, however, is whether the earlier data on empiric treatment are still applicable, now that invasive H influenzae disease has disappeared. Evidence suggests that the old conventional wisdom, which advocated empiric treatment of suspected bacteremia on the basis of concerns about early H influenzae disease, can no longer be supported.
Adverse consequences of empiric treatment
In children who have serious allergic reactions to beta-lactam antibiotics, alternative treatments should be given, including vancomycin or clindamycin. Vancomycin is used in children with serious infections, and clindamycin is given to children with severe hypersensitivities.
In addition, there is significant concern regarding the emerging resistance of common pathogens to beta-lactam antibiotics as a consequence of our overall use of antibiotics in low-risk children. Withholding antibiotics for those children who have fever but do not appear toxic and have no apparent focus of infection is certainly one way of reducing overall use of these agents and thereby helping to reverse the trend of increasing resistance.
Antibiotic Therapy for Confirmed Pneumococcal Bacteremia
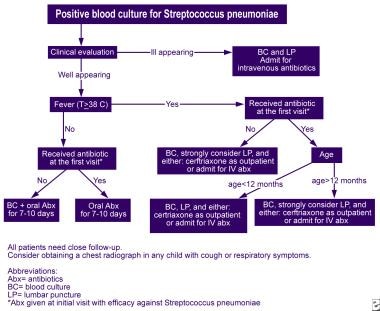
All patients with blood culture results positive for S pneumoniae (pneumococcus) need prompt reevaluation and treatment with antibiotics (see the image below).
Further testing and therapy are determined by the following:
-
Age of patient
-
Condition of patient
-
Persistence of fever
-
Presence of focal infection
On the basis of a large number of recent studies that have observed spontaneous resolution in most cases of occult pneumococcal bacteremia and a low rate of subsequent focal infection, it is now appropriate to provide an immediate follow-up visit and repeat blood culture only to those patients who have become afebrile and have clinically improved after the initial visit (see the table below).
Table 3. Management of Patients With Positive Blood Culture for Streptococcus pneumoniae and No Focus of Infection (Open Table in a new window)
Febrile and/or toxic |
Hospitalization Repeated blood culture Intravenous antibiotics |
Afebrile and asymptomatic |
Repeated blood culture Continued observation at home and follow-up if indicated Oral antibiotics for 10 days Continue antibiotics orally if begun at initial visit |
All patients with significant underlying medical conditions, especially any immunodeficiency, require admission for parenteral antibiotics and close observation. Most patients can be treated as outpatients, but all patients need close follow-up care. Reviewing sensitivities of the isolate in order to tailor subsequent therapy is essential because of the increasing rates of penicillin-resistant pneumococcus.
Patients with proven pneumococcal bacteremia can be treated with penicillins or cephalosporins unless the isolate has specific antibiotic resistance. Because a small percentage of pneumococci are resistant to cephalosporins, all life-threatening infections should be treated with vancomycin in addition to ceftriaxone or cefotaxime, pending results of the antibiotic susceptibilities.
For outpatients with a positive blood culture for pneumococcus, appropriate therapy can be determined at the reevaluation visit. Patients who are afebrile, appear well, and show no evidence of new focal infection at the reevaluation visit can be treated successfully with outpatient oral antibiotics, such as amoxicillin (40-80 mg/kg/day in 3 divided doses).
Prevention
In 2000, a heptavalent pneumococcal conjugate vaccine (PNCV7) was approved for routine administration to young infants. This vaccine covered S pneumoniae types 4, 6B, 9V, 14, 18C, 19F, and 23F, which at that time accounted for 82-94% of documented cases of pneumococcal disease. Administration of PNCV7 resulted in a 90% reduction in the incidence of bacteremia and invasive pneumococcal disease and a 70% reduction in all cases of suspected bacterial pneumonia among recipients. [34, 45, 40] PNCV7 is no longer available on the market.
In the years succeeding the development of PNCV7, the slow emergence of nonvaccine serotypes that caused invasive disease—particularly type 19-A [46, 47, 48] —led to the development and licensure of a new vaccine designed to increase overall protection by covering an additional 6 strains, including types 1, 3, 5, 6A, 7F, and 19A.
In 2010, this 13-valent vaccine (PCV13) was approved by the US Food and Drug Administration (FDA) and recommended for routine administration to children. It is anticipated that the use of the 13-valent vaccine will yield an even greater reduction in pneumococcal disease than was previously achieved with PNCV7. [49, 50] The 15- and 20-valent vaccines were approved for children in 2022 and 2023, respectively.
PCV is indicated for active immunization as follows:
-
PCV13, PCV15, PCV20: Aged 6 weeks through 17 years to prevent invasive disease caused by Streptococcus pneumoniae
-
PCV13, PCV20: Aged 6 weeks through 5 years to prevent otitis media caused by S pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F
PCV13, PCV15, and PCV20 are indicated for active immunization in children 6 weeks through 5 years of age. Four 0.5 mL doses are given intramuscularly, administered at 2, 4, 6, and 12-15 months. The first dose can be given as early as 6 weeks, and the fourth dose should be administered at least 2 months after the third dose.
Pneumococcal vaccine serotypes (Open Table in a new window)
| Vaccine | Serotypes |
|---|---|
| PCV13 (Prevnar 13) | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F |
| PCV15 (Vaxneuvance) | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 22F, 23F, and 33F |
| PCV20 (Prevnar 20) | 1, 3, 4, 5, 6A, 6B, 7F, 8, 9V, 10A, 11A, 12F, 14, 15B, 18C, 19A, 19F, 22F, 23F, and 33F |
Vaccinated children who are 7-11 months of age should receive 3 doses (with the first 2 doses given at least 4 weeks apart and the third dose given after the 1-year birthday, separated from the second dose by at least 2 months). Children who are 12-23 months of age should receive 2 doses that are at least 2 months apart.
Children who previously received one or more doses of PNCV7 may complete immunization with the pneumococcal 13-valent conjugate vaccine. Children 15 months to 5 years of age who are considered completely immunized with PNCV7 may receive one dose of the 13-valent pneumococcal conjugate vaccine.
Prognosis
According to the World Health Organization, at least 6 million children die each year of pneumococcal infections (eg, pneumonia, meningitis, bacteremia); most of these fatalities occur in developing countries. However, the prognosis for uncomplicated pneumococcal bacteremia is excellent with good follow-up and appropriate antibiotic therapy. [51]
Ten percent of patients with pneumococcal bacteremia develop focal complications, primarily otitis media and pneumonia. Meningitis carries the highest risk for significant morbidity and mortality, including a 25-30% risk of neurologic sequelae such as deafness, intellectual disability, seizures, and paralysis. The mortality associated with pneumococcal meningitis is 6.3-15%, and the overall mortality of pneumococcal bacteremia is 0.8%. [25]
Complication rates are higher in immunocompromised patients, including those with malignancy, asplenia (eg, sickle cell disease), and HIV.
Patient Education
Parents of patients who are thought to be at risk for pneumococcal bacteremia should be educated properly with standard fever instructions. Parents should seek further care for any of the following reasons:
-
Worsening condition
-
New symptoms
-
Lethargy
-
Irritability
-
Alteration in mental status
-
Signs of dehydration
-
Severe headache
-
Stiff neck
-
Rash
-
Algorithm for the reevaluation of outpatients with Streptococcus pneumoniae bacteremia.
Tables
What would you like to print?
- Overview
- Epidemiology
- Clinical Presentation and Laboratory Features
- History and Physical Examination
- Laboratory Studies
- Chest Radiography
- Empiric Antibiotic Therapy for Suspected Occult Bacteremia
- Antibiotic Therapy for Confirmed Pneumococcal Bacteremia
- Prevention
- Prognosis
- Patient Education
- Show All
- Tables
- References