Background
Pheochromocytoma is a rare catecholamine-secreting tumor that arises from chromaffin cells of the sympathetic nervous system (adrenal medulla or and sympathetic chain); however, the tumor may develop anywhere in the body "catecholamine-secreting paragangliomas" ("extraadrenal pheochromocytomas"). The term paraganglioma refers to any extra-adrenal or nonfunctional tumor of the paraganglion system, whereas functional tumors are referred to as extra-adrenal pheochromocytomas. Pheochromocytomas and catecholamine-secreting paragangliomas have a similar clinical presentation, but the risk for associated neoplasms, risk for malignancy, and genetic testing is different between the tumors.
Release of catecholamines into the circulation by these tumors causes significant hypertension. The classic clinical presentation includes paroxysmal attacks of headaches, pallor, palpitations, and diaphoresis.
Pheochromocytoma may be inherited as an autosomal dominant trait. Several genes (SDHD, SDHB, SDHC) that belong to the mitochondrial complex II have been identified as involved in the so-called pheochromocytoma-paraganglioma syndrome. (See Etiology.)
Pheochromocytomas occur in both children and adults. [1] In children, pheochromocytoma is more frequently associated with other familial syndromes, such as neurofibromatosis, von Hippel-Lindau disease, tuberous sclerosis, Sturge-Weber syndrome, and as a component of multiple endocrine neoplasia (MEN) syndromes (MEN 2A, MEN 2B). Familial cases are often bilateral or multicentric within an individual adrenal gland.
Adrenal pheochromocytomas are most often found on the right side and are sporadic, unilateral, and intra-adrenal. Approximately 6-10% of the tumors are malignant.
Usually, extra-adrenal tumors (extra-adrenal pheochromocytomas or paragangliomas) are located in the abdomen along the sympathetic chain and constitute about 10% of sporadic cases. Tumors have also been found in the neck, mediastinum, urinary bladder, and virtually every other site. Tumors vary from approximately 1-10 cm in diameter. Slowly growing metastases to bone, liver, lymph nodes, and lung can arise from malignant tumors.
Early diagnosis is important because the tumor may be fatal if undiagnosed, especially in pregnant women during delivery or in patients undergoing surgery for other disorders. Diagnosis can be made based on elevated levels of urinary catecholamines, but localization may require various modalities (see the images below, as well as Workup).
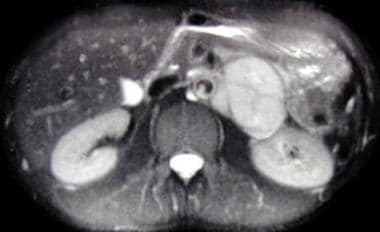 Axial, T2-weighted MRI scan showing large left suprarenal mass of high signal intensity on a T2-weighted image. The mass is a pheochromocytoma.
Axial, T2-weighted MRI scan showing large left suprarenal mass of high signal intensity on a T2-weighted image. The mass is a pheochromocytoma.
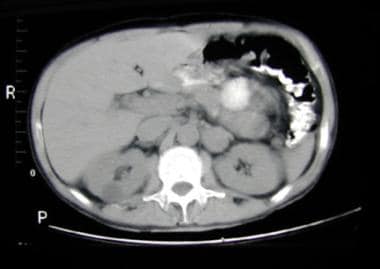 Abdominal CT scan demonstrating left suprarenal mass of soft tissue attenuation representing a paraganglioma.
Abdominal CT scan demonstrating left suprarenal mass of soft tissue attenuation representing a paraganglioma.
Treatment is with surgical removal. Chemotherapy and radiotherapy have been used in metastatic and unresectable pheochromocytoma, but their value is questionable. (See Treatment.)
For a discussion of pheochromocytoma in adults, see the Medscape article Pheochromocytoma.
Pathophysiology
Pheochromocytoma is a tumor of neuroendocrine origin. In the fifth week of fetal development, neuroblastic cells migrate from the thoracic neural crest to form the sympathetic chains and preaortic ganglia. These cells are believed to be the precursors of neuroblastomas and ganglioneuromas.
Chromaffin cells migrate a second time to the adrenal medulla; the chromaffin cells settle near the sympathetic ganglia, the vagus nerve, paraganglia, and carotid arteries. Other, less common sites of extra-adrenal chromaffin tissues include the following:
-
Bladder wall
-
Prostate
-
Behind the liver
-
Hepatic hilum
-
Renal hilum
-
Rectum
-
Gonads
Catecholamine biosynthesis and metabolism
The pathophysiology of the pheochromocytoma is best appreciated with an understanding of catecholamine biochemistry.
The following is an abbreviated version of the important steps in the biosynthesis and metabolism of catecholamines:
Tyrosine → dihydroxyphenylalanine (DOPA) → dopamine (DA) → norepinephrine + epinephrine → homovanillic acid (HVA) + vanillylmandelic acid (VMA)
The biosynthesis and storage of catecholamines in chromaffin cell tumors may differ from the biosynthesis and storage in the normal medulla. However, the granules are morphologically and functionally similar to the granules from the adrenal medulla. The increase in tissue turnover suggests an alteration in the regulation of the catecholamine biosynthesis and possibly suggests an alteration in the feedback inhibition of tyrosine hydroxylase, the key enzyme in the production of catecholamines.
Catecholamines in pheochromocytomas
Pheochromocytomas, unlike the normal adrenal medulla, are not innervated, and catecholamine release is not initiated by neural impulses. Changes in direct flow, pressure, chemicals, drugs, and angiotensin II may initiate the release of catecholamines into the circulation.
Most pheochromocytomas in children predominantly produce norepinephrine, unlike the normal adrenal medulla, which, in humans, contains 85% epinephrine. Rarely, tumors produce epinephrine exclusively; in some cases, the clinical picture is dominated by signs of beta-receptor stimulation, such as tachycardia and hypermetabolism. However, in most cases, predicting the pattern of catecholamine secretion based on the clinical picture is impossible.
The actions of catecholamines are mediated by the alpha-adrenergic and beta-adrenergic receptors. Alpha1 receptors cause arteriolar constriction. Alpha2 receptors mediate the presynaptic feedback inhibition of norepinephrine release and decrease insulin secretion.
Beta1 receptors increase cardiac rate and contractility. Beta2 receptors cause arteriolar and venous dilation and relaxation of tracheobronchial smooth muscle. The symptoms associated with pheochromocytomas are caused by the physiologic and pharmacologic effects of large amounts of circulating norepinephrine and epinephrine.
Pheochromocytomas in patients with von Hippel-Lindau syndrome and MEN type 2 differ in the types and amounts of catecholamines produced and the resulting signs and symptoms. Eisenhofer et al reported that the rate constant for baseline catecholamine secretion was 20-fold higher from tumors in von Hippel-Lindau syndrome (n = 47) than from MEN2 tumors (n = 32), but catecholamine release in response to glucagon occurred only in MEN2 tumors. [2]
Thus, the differences in catecholamine release may contribute to clinical differences in the secretion of neurotransmitters or hormones and the subsequent clinical presentation. [2]
Animal models
Over the last 2 decades, various mouse and rat models have been created presenting with pheochromocytomas, which include models presenting tumors that are to a certain degree biochemically and/or molecularly similar to human pheochromocytomas and develop metastases. In addition, cell lines such as mouse pheochromocytoma (MPC) and mouse tumor tissue (MTT) cells have been introduced and they both showed metastatic behavior. It appears these MPC and MTT cells are biochemically and molecularly similar to some human pheochromocytomas, are easily visualized, and respond to different therapies. [3]
Pheochromocytoma is inducible in rats by various nongenotoxic substances that may act indirectly by stimulating chromaffin cell proliferation. The nerve growth factor-responsive PC12 cell line, established from a rat pheochromocytoma, has served as a research tool for almost 30 years for many aspects of neurobiology involving normal and neoplastic conditions.
Pheochromocytoma cell lines from neurofibromatosis knockout mice supplement the PC12 line and have generated additional applications. [4] Two mice models of metastatic pheochromocytoma have been established: one used tail vein injection of mouse pheochromocytoma cells [5] ; the other involved the conditional knockout of the pten protein. [6] Thus, the use of mouse models allows further study into the pathogenesis of human malignant pheochromocytoma and into therapeutic strategies for these tumors.
Clinical consequences
Bone lesions have been described following changes in the microcirculation.
Myocarditis characterized by focal degeneration and necrosis of myocardial fibers with infiltration of histiocytes, plasma cells, and other signs of inflammation may be present.
Chronic constriction of the arterial and venous beds leads to a reduction in plasma volume. The inability to further constrict the bed upon arising results in postural hypotension.
Etiology
Pheochromocytoma occurs wherever chromaffin tissue is found. Mutations in genes that code for 3 of the 4 components of mitochondrial complex II can cause paragangliomas and pheochromocytomas. The 3 genes involved are SDHB, SDHC, and SDHD.
Of the 4 components of mitochondrial complex II (succinate dehydrogenase [SDH] A, B, C, and D), SDHC and SDHD anchor the catalytic subunits SDHA and SDHB in the inner mitochondrial membrane. SDHD is maternally imprinted, whereas SDHB and SDHC are not. Although SDHD and, to a lesser degree, SDHB mutations have been found in many cases of hereditary paragangliomas, SDHC mutations are rare.
Amar et al delineated causes of pheochromocytoma in a study of 314 patients with pheochromocytoma or functional paraganglioma. [7] Fifty six patients had family history, syndromic disease, or both, and 258 patients had sporadic presentation. Among the 56 patients with a family history, syndromic presentation, or both, 13 had neurofibromatosis type 1, and 43 had germline mutations on the VHL, RET, SDHD, or SDHB genes (16, 15, 9, and 3 patients, respectively).
Only 11% of the patients with sporadic disease had a germline mutation (18 patients had a SDHB mutation, 9 patients had a VHL mutation, 2 patients had a SDHD mutation, and 1 patient had a RET mutation). Mutation carriers were young and frequently had bilateral or extra-adrenal tumors. In patients with an SDHB mutation, the tumors were larger, usually extra-adrenal, and malignant.
Pheochromocytomas are usually sporadic, but they may be familial and appear as a component of other syndromes, such as MEN 2A (medullary thyroid carcinoma, parathyroid hyperplasia, pheochromocytoma). Germline mutations of the ret proto-oncogene on chromosome 10 (10q11.2) have been found in families with MEN 2A and MEN 2B (medullary thyroid carcinoma, neuromas, pheochromocytoma).
In von Hippel-Lindau syndrome, specific mutations determine the varied clinical manifestations, which, in addition to pheochromocytomas, include retinal angiomas; cerebellar hemangioblastomas; and renal, pancreatic, and epididymal tumors. A germline mutation in a tumor suppressor gene on chromosome 3 has been identified.
Pheochromocytoma is also associated with tuberous sclerosis, Sturge-Weber syndrome, and ataxia-telangiectasia.
Epidemiology
In the United States, the reported annual incidence rate of pheochromocytomas is approximately 1 per 100,000 population, with 10-20% of cases occurring in children or adolescents. The frequency of bilateral tumors is higher in children than in adults (20% vs 5-10%), while that of malignant tumors is lower (3.5% vs 3-14%). More than one third of affected children have multiple tumors, most of which are recurrent.
In children, 70% of cases are unilateral, 70% of cases are confined to adrenal locations, and an increased association with familial syndromes is noted. In 30-40% of children with pheochromocytomas, tumors are found in both adrenal and extra-adrenal areas or in only extra-adrenal areas.
Race-, sex-, and age-related demographics
Pheochromocytomas have been described in Japanese, Chinese, black, European, and white families. No geographic predilection is known.
Although pheochromocytomas are found in both sexes, the male-to-female ratio is 2:1. In a study by Lai et al, female patients have significantly more self-reported pheochromocytoma signs and symptoms compared with males; these include the following [8] :
-
Headache (80% vs 52%)
-
Dizziness (83% vs 39%)
-
Anxiety (85% vs 50%)
-
Tremor (64% vs 33%)
-
Weight change (88% vs 43%)
-
Numbness (57% vs 24%)
-
Changes in energy level (89% vs 64%)
In childhood, pheochromocytomas present most frequently in children aged 6-14 years (average, 11 y).
A 2011 study that identified 41 subjects with metastatic pheochromocytomas and compared them with 108 subjects with apparently benign pheochromocytomas showed that metastatic pheochromocytomas presented at a significantly younger age (41.4 ±14·7 y vs 50.2 ±13.7 y; P< .001), were larger (8.38 ±3·27 cm vs 6·18 ±2.75 cm; P< .001), had more frequent secretion of norepinephrine, and had a higher occurrence of necrosis. [9]
Prognosis
The prognosis in patients with pheochromocytomas appears to be related to tumor size, degree of uncontrolled hypertension, and the presence of metastatic disease.
Uncontrolled hypertension may lead to serious, even fatal, morbidity, such as the following:
-
Myocardial infarction
-
Stroke
-
Arrhythmias
-
Irreversible shock
-
Renal failure
-
Dissecting aortic aneurysm
Special consideration must be given to prepare these patients for surgery, because dramatic blood pressure swings may be observed.
Malignant pheochromocytomas, which are rare in children, are locally invasive and may spread to distant areas that do not contain chromaffin cells, including the liver, lung, bone, and lymph nodes. The mean 5-year survival rate in patients with malignant pheochromocytomas is 40%.
A study by Khorram-Manesh et al of the long-term outcome in Swedish patients who underwent surgical treatment of pheochromocytoma from 1950-1997 found that over 15 (±6) years, 42 patients died, compared with 23.6 deaths expected in the general population. [10] Besides older age at primary surgery, elevated urinary excretion of methoxy-catecholamines was the only observed mortality risk factor. Preoperative and postoperative hypertension did not influence the mortality risk compared with controls.
A retrospective study by Timmers et al in the Netherlands documented that metastases, but not cardiovascular mortality, reduced life expectancy in 69 patients who had undergone surgical resection of apparently benign pheochromocytoma. [11] Kaplan-Meier estimates for 5-year and 10-year survival since surgery were 85.8% and 74.2% for patients compared with 95.5% and 89.4% in the reference population.
Two patients died of surgical complications. All 10 patients with metastatic disease died, including 3 diagnosed at first surgery. At follow-up, 40 patients were alive and recurrence-free, and 3 patients were lost to follow up. Two patients experienced a benign recurrence. A significant decrease in blood pressure was observed in 64% of patients with hypertension prior to surgery; however, they remained hypertensive after surgery. [11]
Pheochromocytoma during pregnancy represents a condition with potentially high maternal and fetal mortality. However, in current practice, pheochromocytoma in pregnancy is recognized earlier, and, in conjunction with improved medical management, maternal mortality has decreased to less than 5%. [12]
-
Axial, T2-weighted MRI scan showing large left suprarenal mass of high signal intensity on a T2-weighted image. The mass is a pheochromocytoma.
-
Abdominal CT scan demonstrating left suprarenal mass of soft tissue attenuation representing a paraganglioma.







