Background
The activities of swallowing and speaking depend upon the ability to obtain adequate closure of the velopharyngeal port. Both are complex motor skills that involve the coordination of a diverse group of muscles along the upper aerodigestive tract. Velopharyngeal movements during phonation are quite distinct from those involved in swallowing, as is clinically evident in patients who are able to obtain good closure during swallowing yet are unable to obtain adequate closure when speaking.
Velopharyngeal closure (VPC) is an important part of speech. All phonemes in the English language, with the exception of the three nasal phonemes (/m/, /n/, /ng/), are produced with oral airflow, meaning that the velopharynx should be closed. The nasal phonemes are produced with nasal resonance, meaning that the velopharynx must open during their production. With so many phonemes in English requiring oral airflow, oral resonance is important to production of intelligible speech.
Phonation involves the generation of a column of air pressure passing from the subglottis into the upper airway. If VPC is inadequate, air is allowed to escape through the nose during the generation of consonants requiring high oral pressure, leading to inappropriate nasal resonance during speech production. The consequence of impaired VPC is velopharyngeal dysfunction (VPD), the constellation of speech production disorders that includes velopharyngeal insufficiency (VPI), velopharyngeal incompetence, and velopharyngeal incorrect learning. (See Pathophysiology.)
Causes of hypernasality and VPD are many and range from structural causes (eg, cleft palate) to neuromuscular problems (eg, those observed in velocardiofacial [VCF] syndrome). Functional etiologies also exist, including splinting of the palate after tonsillectomy, imitation of cultural or familial role models, and phoneme-specific problems. (See Etiology.)
Patients with VPD may present with hypernasality, nasal emission, or facial grimacing. In their attempt to be understood, affected patients often develop compensatory maladaptive articulations that are very difficult to reverse if left untreated. (See Clinical Presentation.)
Patients with VPD should be treated within in the context of multidisciplinary team care. (See Treatment.) In 1988, an international working group convened to standardize definitions and assessment methodologies. [1] The working group strongly recommended implementing a multidisciplinary team approach and using multimodal instruments to evaluate preoperative and postoperative speech outcomes.
The group also asserted that comprehensive analysis of specific causes of speech production disorders, through perceptual and instrumental measures of velopharyngeal function, allows for customized treatment algorithms for specific patients.
Definitions of key terms
In describing the problem of hypernasal speech, differentiation between velopharyngeal incorrect learning, velopharyngeal incompetence, and VPI is important. [2] Ideally, care providers representing the various disciplines of the cleft team should use the same nomenclature so that they can effectively organize and communicate their knowledge. [3, 4] Trost-Cardamone developed a useful taxonomy to classify possible causative factors of VPD. [5]
Velopharyngeal incorrect learning is best described as incorrect closure of the velopharyngeal port in the nasopharynx as a result of articulation difficulties; inconsistent hypernasality or phoneme-specific hypernasality is typical of this condition. It may be the result of phoneme-specific nasal emission and deafness or hearing impairment.
Velopharyngeal incompetence is inadequate VPC occurring as a result of neurogenic causes, such as a functional problem with the oral motor mechanism, as may be seen in paresis, apraxia, and dysarthria.
In VPI, there is insufficient tissue to accomplish closure of the velopharyngeal sphincter. The typical cause is a structural problem with the velum (eg, submucous cleft palate, shorted velum relative to the depth of the posterior pharyngeal wall, or overt cleft palate). VPI can also be due to mechanical interferences with closure (eg, excessively large tonsils, webbing of the posterior tonsillar pillars, or both). [6]
As a rule, velopharyngeal incorrect learning, VPI, and velopharyngeal incompetence have approximately the same effect on the patient’s speech. For this reason, these terms are typically used interchangeably or are subsumed under the general heading of VPD.
It is also important to be familiar with the terms used to describe key manifestations of VPD. [7, 8]
Nasalance is an acoustic correlate of nasal resonance. It is calculated as the ratio of nasal to nasal-plus-oral energy.
Nasal airflow (nasal emission) is different from the nasal acoustic energy associated with hypernasality. It involves a nasal, rather than an oral, increase in airflow. Nasal air emission results from an attempt to build up intraoral air pressure for the production of consonants in the presence of a leak in the system, fistula, or incompetent velopharyngeal valve. Some of the airflow is released through the nose, causing a disruption in the aerodynamic process of speech.
Nasal air emission is noted on pressure-sensitive phonemes, plosive fricatives, and affricates (though not on vowels), and it can occur with normal resonance. Whether it is audible depends on the size of the opening: the bigger the opening, the less the resistance to flow. However, a small velopharyngeal gap may often release a paradoxically high stream of airflow pressure, making an audible noise and generating a misleadingly high nasalance score.
Nasal rustle or turbulence is a distracting sound that accompanies consonant production. Generally, small constrictions in the nasopharynx produce a distinctive fricative sound on the voiced pressure consonants b, d, and g.
Hypernasality is produced by nasally escaping air reverberating in a confined postnasal space. Specifically, it is abnormal nasal resonance during the production of nonnasal oral sounds. Hypernasality is a resonance disorder that is usually related to VPD as a result of the lack of a barrier between the oral and nasal cavities, which leads to abnormal coupling (sharing of acoustic energy) of these cavities during speech. Whereas hypernasality usually refers to velopharyngeal sphincteric function, it may be secondary to a fistula or an unrepaired cleft palate.
Grimacing is an aberrant facial muscle movement. It represents a subconscious attempt by the patient to inhibit abnormal nasal airflow by constricting the nares.
Hyponasality is a blocked-up tone that may occur with nasal obstruction from enlarged adenoids, a deviated septum, an inadequate nasal airway, or chronic catarrh.
Go to Surgical Treatment of Velopharyngeal Dysfunction for complete information on this topic.
Anatomy
The velopharyngeal sphincter comprises the following six muscles:
-
Tensor veli palatini
-
Levator veli palatini
-
Uvular muscle (musculus uvulae)
-
Palatoglossus
-
Palatopharyngeus
-
Superior constrictor
The tensor veli palatini arises from the scaphoid fossa, spine of the sphenoid, and the cartilaginous portion of the eustachian tube. It inserts into a tendon winding around the hamular process. Innervated by the mandibular branch of cranial nerve V, it tenses the soft palate and opens the eustachian tube during swallowing.
The levator veli palatini arises from the petrous apex and the cartilaginous portion of the eustachian tube. Its fibers fan out in the soft palate and blend with the contralateral levator. Innervated by the pharyngeal plexus from cranial nerves IX and X, it pulls the velum in a posterosuperior direction and serves as the major elevator for the velum.
The uvular muscle arises from the palatal aponeurosis posterior to the hard palate and inserts into the uvula mucosa. Innervated by the pharyngeal plexus, it functions to add bulk to the dorsal aspect of the uvula.
The palatoglossus arises from the anterior surface of the soft palate and inserts into the lateral aspect of the tongue base. Innervated by the pharyngeal plexus, it simultaneously lowers the velum and elevates the tongue upwards and backwards. It depresses the palate for nasal speech.
The palatopharyngeus arises from the soft palate and inserts into the posterior border of the thyroid cartilage. Innervated by the pharyngeal plexus, it positions the velum and narrows the velopharyngeal orifice by adducting the posterior pillars and constricting the pharyngeal isthmus. This muscle also raises the larynx and lowers the pharynx.
The superior constrictor arises from the lower portion of the pterygoid plate and the hamular process and inserts into the median raphe. Innervated by the pharyngeal plexus, it produces medial movement of the pharyngeal walls and assists in drawing the velum posteriorly. The inferior portion of this muscle forms the Passavant ridge. Early descriptions of speech described contact of the soft palate with this fold of the posterolateral pharyngeal wall. However, newer observations suggest that the soft palate contacts the posterior nasopharyngeal wall above the level of this ridge.
On endoscopy, the velopharynx (nasopharynx) has several topical anatomic features that are important to note.
-
Pharyngeal ostium of the eustachian (auditory) tube
-
Pharyngeal tonsil (ie, adenoid)
-
Posterior pharyngeal wall
-
Pharyngeal surface of the soft palate
The pharyngeal ostium of the eustachian (auditory) tube and its associated structures make up the lateral wall of the nasopharynx. The ostium is bordered anteriorly by the salpingopalatine fold and posteriorly by the cartilaginous torus tubarius. Posterior to the torus tubarius is the pharyngeal recess. Extending inferiorly from the torus tubarius is the salpingopharyngeal fold, which overlies the salpingopharyngeal muscle. Inferior to the ostium and torus, the pterygopharyngeal portion of the superior pharyngeal constrictor muscle makes up the lateral wall of the nasopharynx.
The adenoid, a closely aggregated collection of lymphoid nodules, is directly posterior in the nasopharynx and overlies the basilar portions of the sphenoid and occipital bones. It lies directly between the tori tubarii on either side and may extend laterally into the pharyngeal recess, where it is commonly known as the eustachian or Gerlach tonsil. Superiorly, it may extend up into the nasal choanae; inferiorly, it ends at or near the level of the anterior arch of the atlas. The prevertebral fascia and the atlanto-occipital membrane lie deep to the pharyngeal tonsil.
The posterior pharyngeal wall, which is immediately inferior to the pharyngeal tonsil, comprises mainly the superior pharyngeal constrictor muscle.
The pharyngeal surface of the soft palate is formed by the aponeuroses of several muscles, including the levator veli palatini muscle, the tensor veli palatini, the palatopharyngeus, and the uvular muscle (see above). The insertion and innervation of these muscles is described above.
Pathophysiology
Oral resonance (as contrasted to nasal resonance) is obtained by means of VPC, which creates a seal between the nasopharynx and the oral cavity. Typically, VPC is accomplished by elevation of the velum and approximation of the lateral walls to close off the nasopharynx. In a small group of patients, formation of a Passavant ridge on the posterior pharyngeal wall may contribute to closure.
Four basic types of closure patterns are used to describe velar closure: coronal, sagittal, circular, and circular with the Passavant ridge (see the image below). Defining the type of closure pattern is important when surgical intervention for correction of VPD is under consideration.
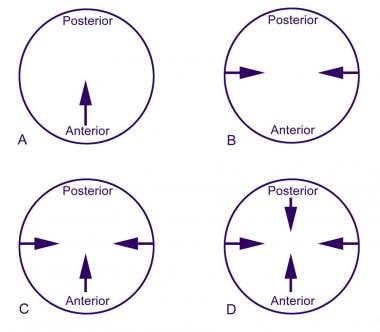 Pharyngeal closure patterns: (A) coronal, (B) sagittal, (C) circular, and (D) circular with Passavant ridge.
Pharyngeal closure patterns: (A) coronal, (B) sagittal, (C) circular, and (D) circular with Passavant ridge.
Coronal closure, the most common closure pattern, is present in 55% of patients with normal velar function. The major contribution to closure is from the soft palate as it contacts a broad area of the posterior pharyngeal wall. Little medial motion of the lateral pharyngeal walls occurs. A coronal closure pattern is often present with an enlarged adenoid pad.
Sagittal closure, the least common pattern, is present in 10-15% of people. Here, palatal elevation is minimal. The main contribution is from medial motion of the lateral pharyngeal walls. This is the pattern seen most commonly in patients with persistent VPD after repair of a cleft palate.
Circular closure, which is present in approximately 20% of individuals with normal closure, involves contributions from both the soft palate and the lateral pharyngeal walls. This results in a closure that resembles a circle getting smaller.
Circular closure with the Passavant ridge, which occurs in 15-20% of the population, is a circular pattern that involves anterior motion of the posterior pharyngeal wall (known as Passavant’s ridge).
VPD is what happens when VPC is impaired. The effects of VPD on a patient’s speech include hypernasality, decreased speech intelligibility, and nasal emissions (ie, air escape out of the nose during speech). Several factors determine how severely a patient’s speech is affected, including the amount of gap with a closed velum, the patient’s articulation and oral motor ability, and compensatory strategies the patient may have developed to decrease nasal emission or hypernasality.
Common compensatory strategies include speaking with soft intensity (ie, volume) to decrease airflow through the nasal cavity, speaking with loud intensity or pushing to try to project the voice, and substituting phonemes that require less airflow. Straining to increase oral airflow often results in vocal nodules, and hoarseness is a common finding with VPD.
Etiology
The etiology of VPD includes structural abnormalities of the palate, dynamic impairment of a structurally normal palate, and functional abnormalities that are unassociated with anatomic or dynamic palatal defects. The following is an overview of the most common causes.
Palatal and other physical causes
Overt cleft palate, either before or after repair, is by far the most common cause of VPD, occurring in approximately 1 of every 2000 live births. VPD has been reported in as many as 30-50% of patients after palate repair. Studies have shown that children born with a complete cleft palate are more likely to require VPI surgery than children with incomplete clefts.
A submucous cleft palate is defined by the presence of a bifid or double uvula, muscular diastasis of the soft palate, and notching of the posterior border of the hard palate. This is usually evident on examination of the oral cavity, especially with elevation of the palate when the patient pronounces the phoneme /ah/.
By contrast, an occult submucous cleft palate is an absence or deficiency of the musculus uvulae with a diastasis of the levator veli palatini but without the presence of a bifid uvula or grooving of the oral surface of the soft palate. An occult submucous cleft is best visualized endoscopically as the absence of the bulge and the presence of a central groove on the nasal surface of the soft palate during speech.
Most patients with either overt or occult submucous cleft palate can produce normal speech. However, because of their abnormal musculature, these patients may be predisposed to VPD by any changes to the velopharyngeal anatomy, such as those occurring as a result of adenoidectomy or velocardiofacial syndrome.
VPD may be caused by tonsil hypertrophy that prevents the palate from adequately moving superiorly during attempted velopharyngeal closure. Tonsillectomy may be appropriate in these patients.
Syndromic causes
Patients with trisomy 21 (ie, Down syndrome) are predisposed to VPD. The combination of oromotor and developmental delays, generalized hypotonia, and intellectual delays constitutes a significant risk factor for VPD, especially after adenoidectomy.
VCF syndrome is an autosomal dominant disorder linked to microdeletions in the long arm of chromosome 22. Major findings include cleft palate (overt, submucous, or occult submucous), conotruncal heart anomalies, frequent infections (as part of DiGeorge syndrome), and a characteristic facial appearance with asymmetry of the lower lip (see the image below).
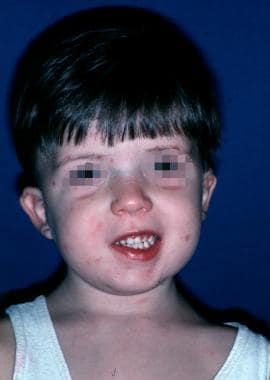 Child with velocardiofacial syndrome. Characteristic clinical findings included unilateral lower lip palsy, small bulbous nose, wide palpebral fissures, and small external ear canals. Patient's speech has hypernasal resonance.
Child with velocardiofacial syndrome. Characteristic clinical findings included unilateral lower lip palsy, small bulbous nose, wide palpebral fissures, and small external ear canals. Patient's speech has hypernasal resonance.
VPD is common in patients with VCF syndrome, not only because of the presence of a cleft palate, but also because of pharyngeal hypotonia. [9, 10, 11, 12] VPI has been reported to be present in approximately 75% of patients with 22q11 deletions, with only 10% of those patients showing actual submucous clefts, providing credence to the theory that VPI occurs in patients with either anatomic or syndromic (ie, functional) causes.
VPD is also a common feature in Kabuki syndrome (KS). Many findings of Kabuki syndrome are similar to those of VCF syndrome, including cleft palate, cardiac abnormalities (typically coarctation of the aorta), muscular hypotonia, and characteristic facial features. As in patients with VCF syndrome, poor muscular tone of the velopharynx is a major cause of VPD in patients with KS.
Velopharyngeal incompetence or VPI may also be a feature of other syndromes, such as neurofibromatosis, myotonic dystrophy, or any syndrome in which low muscle tone is a feature. The importance of syndrome recognition in patients with VPI is critical, as this population may be at particular risk for postoperative airway obstruction, respond less reliably to surgical correction, and require more aggressive adjunctive speech therapy.
Postoperative causes
Transient VPD with hypernasal resonance after adenoidectomy, with or without tonsillectomy, is not uncommon. This condition may persist for several days to weeks and usually resolves spontaneously. Some nasal regurgitation of liquids may be present during this period. Incidence of persistent velopharyngeal dysfunction after adenoidectomy has been reported to range from 1 per 1500 to 1 per 10,000 patients.
Although the adenoid pad is not necessary for normal VPC, it may assist in closure in children with structural or functional abnormalities of the soft palate. Children at risk of developing persistent VPD after adenoidectomy can often be preoperatively identified by presence of repaired cleft palate, submucous cleft palate, 22q11 deletion, palatopharyngeal disproportion (ie, abnormally deep pharynx), or palatal hypotonia.
Other acquired causes
VPD may develop in children with a stroke or head injury, especially if damage occurs to the motor centers that control the cranial nerves responsible for pharyngeal muscle control or to higher centers controlling complex motor tasks.
Neurologic diseases, such as muscular dystrophy, multiple sclerosis, or brain herniation due to Arnold-Chiari malformation, may result in VPD. Traction on the lower cranial nerves results in paresis of the pharyngeal musculature.
Although primarily seen in adults, amyotrophic lateral sclerosis (ALS) and Parkinson disease may lead to VPD in the more advanced stages of disease.
Children or adults with apraxia or dysarthria may experience VPD and nasal emissions.
Epidemiology
Because VPD is multifactorial, the frequency is unknown. For functional causes, determining the frequency is impossible, because some etiologies are acquired and some are congenital. Structural causes of VPD due to cleft palate occur in 1 in 2000 live births. Almost half of all cleft palates have a syndromic cause; the most common syndrome is VCF syndrome (del 22q11.2). [13] VPD occurs in approximately 20% of children who undergo palatoplasty. [14]
Prognosis
Several studies have been published in support of each of the available options for management of VPD; however, most of the data have not been validated by large numbers of patients, nor have these results been subjected to critical analysis. Most of these studies lack a multidisciplinary evaluation, standardized evaluation and treatment criteria, and methods for assessing surgical outcome.
For example, there are several different types of sphincter pharyngoplasties, though they are often grouped together as if they were the same. These procedures differ in terms of transposition of the flaps, use of muscle tissues, levels of insertion, and whether a synchronous pharyngeal flap is used. Other uncontrolled variables include the status of the tonsils and whether a full-thickness transverse cut is made in the posterior pharyngeal wall mucosa. This heterogeneity of sphincter pharyngoplasties explains some of the difficulty in describing postoperative outcomes.
Cleft palate populations, migratory patterns of treating physicians, and dogmatism among surgeons regarding the best technique are all inherently unstable. Additionally, the study designs often do not include rigorous documentation of the preintervention, peri-intervention, and postintervention states or the methodology for evaluation of the intervention.
Achieving a high compliance rate from a patient population stratified for age, sex, socioeconomic factors, and number of surgical interventions is an arduous task. The outcome assessment instrument must be designed to allow analysis of intrarater and interrater reliabilities of all the extramural raters and, at the same time, not be so cumbersome and burdensome as to reduce compliance.
In the future, outcome reporting based on clinical evidence will probably set the standards for cleft surgery. Surgeons who help manage VPD will be judged by the evidence that their interventions are—or are not—doing the most good for the most people at a price that patients and/or insurance agencies are willing to pay.
Evidence-based practice is the integration of the best research evidence with clinical expertise and patient values. The best evidence comes from randomized clinical trials, which are expensive, time-consuming, and not always possible. Sometimes we must settle for good evidence, which may be the best that is available. Nevertheless, the future demands good evidence, and as cleft surgeons and researchers, we need to supply that evidence.
-
Coronal closure.
-
Sagittal closure.
-
Circular closure.
-
Circular closure with Passavant's ridge.
-
Patient with severe articulation disorder and velocardiofacial syndrome. Little or no velar closure is noted on nasopharyngoscopy ("black hole"). Surgical treatment is with wide pharyngeal flap. Aberrant carotid arteries coursing through nasopharynx complicate surgical management.
-
Infant born with heart murmur, submucous cleft palate, and lower lip asymmetry. Clinical findings are consistent with velocardiofacial syndrome.
-
Child with velocardiofacial syndrome. Characteristic clinical findings included unilateral lower lip palsy, small bulbous nose, wide palpebral fissures, and small external ear canals. Patient's speech has hypernasal resonance.
-
Pharyngeal closure patterns: (A) coronal, (B) sagittal, (C) circular, and (D) circular with Passavant ridge.
-
Coronal closure noted. Primary movement is palate contacting posterior pharyngeal wall, with minimal or no movement of lateral pharyngeal walls. Notice air escape along lateral margins as palate contacts adenoid pad.
-
Sagittal closure demonstrated. Soft palate moves little, with most of the closure achieved by movement of lateral pharyngeal walls. Notice notching of soft palate consistent with submucous cleft palate.
-
Example of circular closure with contributions from palate and lateral pharyngeal walls. Patient underwent recent adenoidectomy as evidenced by nasopharyngeal eschar.
-
Circular closure with Passavant ridge demonstrated. Note elevation of ridge on posterior pharyngeal wall contributing to closure.
-
Case study of patient status post cleft palate repair. Child presents with hypernasal speech, especially with /s/. No history of nasopharyngeal reflux. Intraoral examination demonstrates repair to be intact except for posterior most portion (bifid uvula is noted). Nasopharyngoscopy demonstrates notching of soft palate (arrow) and enlarged adenoid pad (asterisk).
-
Velar closure noted as patient pronounces /s/. Circular closure pattern is noted with central defect and air escape. Palate closure is noted with swallow in middle portion of frame.
-
Palate closure noted against adenoid pad as the patient speaks /p/. Phoneme-specific velopharyngeal dysfunction is diagnosed, and speech therapy is recommended to improve articulation. Adenoidectomy in this patient would most likely result in structural velopharyngeal insufficiency.
-
Palatal lift. Hard and soft palatal components are shown.
-
Palatal lift in situ.
-
Preoperative nasoendoscopic view of velopharynx, showing nasal septum (1), lateral nasoparyngeal wall (2, 4), and velum (3).






