Background
Omphalitis is an infection of the umbilical stump. [1] It typically presents as a superficial cellulitis that can spread to involve the entire abdominal wall and may progress to necrotizing fasciitis, myonecrosis, or systemic disease. Omphalitis is uncommon in industrialized countries outside the setting of umbilical vessel catherization; however, it remains a common cause of neonatal mortality in less developed areas. It is predominantly a disease of the neonate, with only a few cases having been reported in adults. Risk factors for omphalitis included septic delivery, unplanned home delivery, maternal chorioamnionitis, prolonged rupture of membranes, low birth weight, and umbilical vessel catheterization.
Aerobic bacteria are present in approximately 85% of infections, predominated by Staphylococcus aureus, group A Streptococcus, Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. [2, 3, 4, 5] Methicillin-resistant S aureus has also been described in association with omphalitis. [6] In the past, studies emphasized the importance of gram-positive organisms (eg, S aureus and group A Streptococcus) in the etiology of omphalitis. This was followed by a series of reports that highlighted the role of gram-negative organisms in the etiology of omphalitis. These studies suggested that the change in etiology may have been caused by the introduction of prophylactic umbilical cord care using antistaphylococcal agents, such as hexachlorophene and triple dye (a widely adopted practice in the 1960s), with a subsequent increase in gram-negative colonization of the umbilical stump.
More recent reports implicate both gram-positive and gram-negative bacteria in the etiology of omphalitis. In some cases, anaerobic bacteria have been found. [7] Many cases are polymicrobial in origin. In some settings, application of herbal and other poultices, human milk, animal dung, ash, etc, may lead to contamination with pathogenic bacteria, including Clostridium tetani.
In addition to monitoring trends in incidence, monitoring the microbial etiology of omphalitis is important, as there have been trends toward returning to dry cord care in most settings, with application of topical antiseptic agents reserved for infants delivered in nonhygenic environments and in locales where neonatal mortality is high. This trend has been widely accepted, including by the World Health Organization (WHO) and the American Academy of Pediatrics (AAP). [8, 9]
Pathophysiology
The umbilical cord connects the fetus to the mother in utero. Composed of connective tissue and blood vessels, the cord is cut immediately after birth, leaving the umbilical stump. Normally, the cord area is colonized with potential bacterial pathogens during or soon after birth. These bacteria attract polymorphonuclear leukocytes to the umbilical cord. Although the precise mechanisms of umbilical cord separation are unknown, granulocyte influx and phagocytosis, as well as desiccation, tissue infarction and necrosis, and the activity of collagenase and other proteases, all contribute to the process.
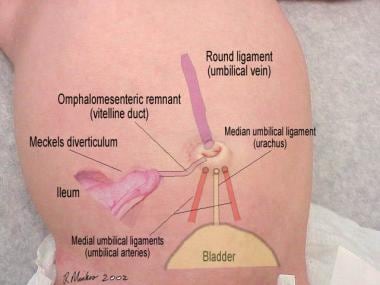
The umbilical stump represents a unique, but universally acquired, wound that, as the tissue undergoes devitalization, provides a medium that supports the growth of bacteria. These bacteria have the potential to invade the umbilical stump, leading to omphalitis. If this occurs, the infection may progress beyond the subcutaneous tissues to involve fascial planes (necrotizing fasciitis), abdominal wall musculature (myonecrosis), and, when the bacteria invade the umbilical vessels, the umbilical and portal veins (phlebitis). The factors that cause colonization to progress to infection are not well understood. The image below shows the anatomic relationship between the umbilicus and its embryologic attachments.
Etiology
Omphalitis is a polymicrobial infection typically caused by a mixture of aerobic and anaerobic organisms.
Associated risk factors include the following:
-
Low birth weight (< 2500 g)
-
Prior umbilical catheterization
-
Septic delivery (as suggested by premature rupture of membranes, nonsterile delivery, or maternal infection)
-
Prolonged rupture of membranes
Omphalitis occasionally manifests from an underlying immunologic disorder. Leukocyte adhesion deficiency (LAD) is most prominent among the immunodeficiency syndromes. [10, 11, 12, 13, 14, 15, 16] Numerous infants with acute or chronic omphalitis have been diagnosed with LAD, a rare immunologic disorder with an autosomal recessive pattern of inheritance. These infants typically present with the following:
-
Leukocytosis
-
Delayed separation of the umbilical cord, with or without omphalitis
-
Recurrent infections
Omphalitis may also be the initial manifestation of neutrophil disorders in the neonate, including neonatal alloimmune neutropenia and congenital neutropenia. [17, 18, 19, 20] Affected infants may present with other cutaneous infections, pneumonia, sepsis, and meningitis.
Neonatal alloimmune neutropenia is a disease analogous to Rh-hemolytic disease and results from maternal sensitization to fetal neutrophils bearing antigens that differ from the mother's. [21] Maternal immunoglobulin G antibodies cross the placenta and result in an immune-mediated neutropenia that can be severe and last for several weeks to 6 months. [22]
The congenital neutropenias are a disease group of heterogeneous disorders that range from intermittent to persistent manifestations of varying severity. [23]
Because omphalitis complicated by sepsis can also be associated with neutropenia, the underlying immune-mediated neutrophil destruction may not be immediately appreciated in affected newborns.
Rarely, an anatomic abnormality such as a patent urachus, a patent omphalomesenteric duct, or a urachal cyst may be present. [24, 25, 26, 27, 28]
Epidemiology
International data
The overall incidence of omphalitis varies from 0.2% to 0.7% in industrialized countries. [29] Its incidence is higher in hospitalized preterm infants than in full-term infants. Omphalitis is usually sporadic but, rarely, epidemics occur (eg, due to S aureus or group A Streptococcus). [30, 31, 32]
Sex- and age-related demographics
No sex predilection has been reported, although males may have a worse prognosis than females.
In full-term infants, the mean age at onset is 5-9 days. In preterm infants, the mean age at onset is 3-5 days.
Prognosis
The prognosis for infants with omphalitis varies.
Outcome is usually favorable in infants with uncomplicated omphalitis associated with cellulitis of the anterior abdominal wall. In a study by Sawin and colleagues, no deaths occurred among 32 infants with omphalitis in the absence of necrotizing fasciitis and myonecrosis. [33] The mortality rate among all infants with omphalitis, including those who develop complications, is estimated at 7%-15%. The mortality rate is significantly higher (38%-87%) after the development of necrotizing fasciitis or myonecrosis. Suggested risk factors for poor prognosis include male sex, prematurity or being small for gestational age, and septic delivery (including unplanned home delivery); however, data are limited and conclusions cannot be drawn regarding the role of these factors in the mortality rate.
Complications
The sequelae of omphalitis may be associated with significant morbidity and mortality. These include necrotizing fasciitis; myonecrosis; sepsis; septic embolization; and, particularly, endocarditis and liver abscess formation, abdominal complications (eg, spontaneous evisceration, peritonitis, bowel obstruction, abdominal or retroperitoneal abscess, abscess of the falciform ligament), and death. [34, 35, 36, 37]
Necrotizing fasciitis
This is a florid bacterial infection of the skin, subcutaneous fat, and superficial and deep fascia that complicates 8%-16% of cases of neonatal omphalitis. [38, 39, 40, 41, 42, 43, 44] It is characterized by rapidly spreading infection and severe systemic toxicity. Necrotizing fasciitis typically involves the abdominal wall but may also involve the scrotum or penis.
Necrotizing soft-tissue infections are caused by production of factors (by single or multiple organisms) that lead directly to tissue cell death, enzymatic destruction of supporting connective tissue, and destruction of host humoral and cellular immune responses to infecting organisms.
Certain organisms are well known to invade tissue and proliferate in necrotic areas. Group A Streptococcus, S aureus, and Clostridium species may elaborate extracellular enzymes and toxins that can damage tissue, may facilitate movement of organisms through soft-tissue planes, and may limit host defenses and penetration of systemic antimicrobial agents. [3]
Myonecrosis
This refers to infectious involvement of muscle.
In infants with omphalitis, development of myonecrosis usually depends on conditions that facilitate the growth of anaerobic organisms. These conditions include the presence of necrotic tissue, poor blood supply, foreign material, and established infection by aerobic bacteria such as staphylococci or streptococci. C perfringens, in particular, does not replicate under conditions of an oxidation-reduction potential (Eh) greater than -80 mV; the Eh of healthy muscle is 120-160 mV. In infections with mixtures of facultative aerobes and anaerobes, the aerobic organisms use oxygen available in tissue, thereby further reducing the Eh in tissues inoculated by Clostridium species or other anaerobic bacteria, often to less than -150 mV, allowing anaerobic bacterial growth.
The toxins produced in the anaerobic environment of necrotic tissue allow rapid spread of organisms through tissue planes. Local spread of toxins extends the area of tissue necrosis, allowing continued growth of organisms and increasing elaboration of toxins. Because of progressive deep tissue destruction and subsequent systemic spread of toxins, anaerobic infections may be fatal if not treated promptly. In addition, rapid development of edema, which constricts the muscle within its fascia, may lead to ischemic myonecrosis.
Sepsis
This is the most common complication of omphalitis. In a study by Mason and colleagues, bacteremia was a complication in 13% of infants with omphalitis. In these infants, shock, disseminated intravascular coagulation (DIC), and multiple organ failure may occur. [4]
Septic embolization
If septic embolization arises from infected umbilical vessels, it may lead to metastatic foci in various organs, including the heart, liver, lungs, pancreas, kidneys, and skin.
Abdominal complications
Abdominal complications include spontaneous evisceration, peritonitis, bowel obstruction, and abscess of the abdomen, retroperitoneum, liver, or falciform ligament.
Long-term or late complications of omphalitis
These may include nonneoplastic cavernous transformation of the portal vein, portal vein thrombosis, extrahepatic portal hypertension, and biliary obstruction. [45, 46, 47] When extrahepatic portal hypertension occurs, gastric or esophageal varices may develop, predisposing to upper gastrointestinal bleeding.
-
Anatomic relationship between the umbilicus and its embryologic attachments.
-
A case of omphalitis (left) associated with extensive myonecrosis (right).
-
A case of omphalitis associated with bullous impetigo due to Staphylococcus aureus.