Practice Essentials
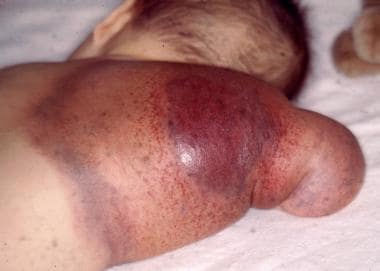
The combination of giant hemangioma, thrombocytopenia, and consumption coagulopathy is termed Kasabach-Merritt syndrome (KMS). KMS is an infrequent but potentially fatal complication of rapidly growing vascular lesions in infants. [1, 2] See the image below.
Signs and symptoms of Kasabach-Merritt syndrome
Signs and symptoms in KMS include the following:
-
Visible cutaneous giant hemangioma or multiple smaller hemangiomas, usually on the extremities
-
Enlarged abdomen
-
Hepatomegaly or jaundice
-
Petechiae, bruising, and frank bleeding
-
Painful lesions
-
Anemia
Physical findings may include the following:
-
Cutaneous hemangioma, often appearing as a large irregular bruise anywhere on the body and often circumscribed by widespread, overlying, shiny and dusky, purple skin
-
Kaposiform hemangioendothelioma (KHE) or tufted angioma (TA) - Blue or reddish-brown discoloration and skin induration
-
Petechiae and bruising
-
Painful, tender lesions
-
Bleeding from thrombocytopenia and coagulopathy (locally and, at times, distantly [disseminated intravascular coagulation (DIC)])
-
Tachycardia, feeding difficulty, and shock (signs of high-output cardiac failure)
-
Pallor (suggestive of anemia)
See Presentation for more detail.
Diagnosis of Kasabach-Merritt syndrome
Any infant with unexplained thrombocytopenia, with or without evidence of DIC, should be evaluated for visceral or hidden vascular lesions (especially of the liver or spleen).
Laboratory studies that may be helpful include the following:
-
Complete blood count (CBC) with differential, reticulocyte count, platelet count, and peripheral smear examination
-
Prothrombin time (PT) and activated partial thromboplastin time (aPTT) - These are prolonged in patients with significant DIC
-
Fibrinogen, fibrin degradation product (FDP), and D-dimer levels - The first are reduced in DIC, and the second and third are elevated
-
Blood culture (to exclude sepsis)
-
Chromosome tests (to exclude certain genetic syndromes)
Diagnostic imaging is obtained as appropriate and may include the following:
-
Radiography
-
Computed tomography (CT)
-
Magnetic resonance imaging (MRI)
-
Doppler flow studies
Radionuclide scanning
Although formal staging is not usually performed, documenting the extent of the invasion of the hemangioma into normal tissue is important for possible subsequent treatment with surgery or radiation. Hemangiomas do not metastasize.
See Workup for more detail.
Management of Kasabach-Merritt syndrome
No single pharmacologic therapy has been proved most effective in patients with KMS. Agents that have been tried (most of them not specifically FDA-approved for this application), with varying success, include the following:
-
Corticosteroids (most commonly used)
-
Interferon alfa
-
Aminocaproic acid (to treat bleeding)
-
Aspirin
-
Dipyridamole
-
Ticlopidine
-
Pentoxifylline
-
Cryoprecipitate
-
Heparin
-
Vincristine (80% response reported)
-
Cyclophosphamide
-
Actinomycin D
-
Propranolol (unlike in infantile hemangioma, response is poor)
Nonpharmacologic treatment modalities include the following:
-
Surgical resection (when lesions are not too large or surgically inaccessible) - Wide local excision is recommended but may be difficult; amputation may be necessary for intractable lesions involving a limb
-
Interventional radiologic procedures (when surgical treatment is not feasible)
-
Intermittent pneumatic compression (most useful for a vascular lesion located on an extremity)
-
Radiation therapy (now largely abandoned because of long-term adverse effects)
See Treatment and Medication for more detail.
Background
The Kasabach-Merritt syndrome (KMS) was first described in 1940 in a male infant with a large, rapidly enlarging, discolored lesion on his thigh that was associated with consumption coagulopathy and thrombocytopenia. The lesion in this original case was a kaposiform hemangioendothelioma, not a classic infantile hemangioma. The combination of enlarging vascular lesion (either a kaposiform hemangioendothelioma or a tufted angioma), profound thrombocytopenia, microangiopathic hemolytic anemia, and consumption coagulopathy is termed KMS or Kasabach-Merritt phenomenon (KMP). [3, 4, 1, 2]
The vascular lesion may be an obvious superficial lesion or a lesion within a visceral organ or even within the brain. Thrombocytopenia is often severe (ie, platelet count < 50,000/µL). Thrombocytopenia and consumption coagulopathy are not complications of all vascular lesions, and size alone does not determine which vascular lesions are associated with thrombocytopenia and coagulopathies. KMS is infrequent but has a potentially fatal outcome (reported at 40% in one report) and is characterized by rapid growth of the vascular lesions in infants. [5] Infantile hemangiomas, the most common tumors of infancy, typically undergo rapid postnatal growth for several months, followed by a prolonged phase of involution.
Pathophysiology and Etiology
The thrombocytopenia associated with vascular lesions is caused by a localized consumption coagulopathy. The vascular lesion triggers an intravascular coagulation with platelet trapping, consequent thrombocytopenia, and fibrinogen consumption and degradation, as well as activation and consumption of coagulation factors, resulting in disseminated intravascular coagulation (DIC). [6] Activation of platelets also promotes further growth of vascular tissue.
KMS is associated with the two following rare vascular lesions::
-
Tufted angioma (TA) [9]
The phenomenon may be associated with trauma in the area of the vascular lesion.
In a study by Ji et al, multivariate analysis indicated that in patients with kaposiform hemangioendothelioma, risk factors for the presence of Kasabach-Merritt syndrome (KMS) include patient age under 6 months, a lesion size of over 5.0 cm, and the existence of a mixed lesion. [10]
Epidemiology
United States and international statistics
Hemangiomas are common vascular tumors that occur in as many as 2.5% of neonates in the United States. Most are benign, and 70-80% regress by the age of 7 years. Some hemangiomas are life-threatening; 1 hemangioma in 300 is associated with coagulopathy. [11] Worldwide, reports of KMS are rare.
Age-, sex-, and race-related demographics
KMS typically occurs in early infancy (< 1 year) or childhood, though prenatal cases (diagnosed with the aid of ultrasonography), newborn presentations, and rare cases in older children and adults have been reported. KMS should be included in the differential diagnosis of unexplained chronic thrombocytopenia at any age.
Boys and men are affected slightly more often than girls and women are. In this respect, KMS differs notably from hemangioma of infancy (infantile hemangioma), which affects girls much more often than it does boys. No racial predilection has been identified.
Prognosis
When KMS is promptly recognized and properly treated, the prognosis is usually excellent because the DIC resolves as the vascular lesion recedes and because KMS does not recur. Therefore, most children do well if they reach age 2 years. When KMS goes untreated, mortality is 10-37%, primarily due to bleeding secondary to the consumption coagulopathy. [11] Even after failed treatment, however, the hematologic abnormalities may spontaneously resolve over a few months.
Mortality and morbidity are influenced by the anatomic location, depth, and extent of the vascular lesion. Besides bleeding, other conditions associated with morbidity and mortality are as follows:
-
Visceral involvement (particularly in the retroperitoneal area and mediastinum)
-
Profound thrombocytopenia
-
DIC
-
Severe infection
-
Iatrogenic complications (eg, from procedures such as arterial ligation, arterial embolization, or surgical excision)
Hemolytic anemia resulting from physical damage to the red blood cells (RBCs) may be mild, moderate, or severe. The results of the direct antibody test (DAT) or the Coombs test is negative in patients with anemia, and anemia is secondary to microangiopathic destruction of the RBCs, [12] manifested by the presence of schistocytes on peripheral blood smear examination (a helpful tool in suggesting the presence of viseral hemangiomas).
Heart failure often occurs in affected infants as a result of the large volume of blood flowing through the giant hemangioma. [13]
An association of KMS with trisomy 21 mosaicism is uncommon; however, 43% of children with Down syndrome have cutaneous vascular lesions. One child with Down syndrome and KMS has been reported. [14]
-
Leg with a Kaposiform hemangioendothelioma, lesion associated with Kasabach-Merritt Syndrome.
-
Back of an arm showing the typical bruising associated with Kasabach-Merritt Syndrome.