Practice Essentials
During normal abdominal development, the 3 divisions of the GI tract (ie, foregut, midgut, hindgut) herniate out from the abdominal cavity, where they then undergo a 270º counterclockwise rotation around the superior mesenteric vessels. Following this rotation, the bowels return to the abdominal cavity, with fixation of the duodenojejunal loop to the left of the midline and the cecum in the right lower quadrant.
Intestinal malrotation, also known as intestinal nonrotation or incomplete rotation, refers to any variation in this rotation and fixation of the GI tract during development. Interruption of typical intestinal rotation and fixation during fetal development can occur at a wide range of locations; this leads to various acute and chronic presentations of disease. The most common type found in pediatric patients is incomplete rotation predisposing to midgut volvulus, requiring emergent operative intervention. [1, 2]
The first reports of intestinal malrotation were based on surgical and autopsy findings and occurred prior to 1900; however, the first description of the embryologic process of intestinal rotation and fixation was not published until 1898. [3] In 1923, Dott was the first to describe the relationship between embryologic intestinal rotation and surgical treatment. [4] In 1936, William E. Ladd wrote the classic article on treatment of malrotation. His surgical approach, now known as the Ladd procedure, remains the cornerstone of practice today. [5]
Pathophysiology
Intestinal malrotation occurs due to disruption of the normal embryologic development of the bowel. Understanding of normal abdominal development aids in the understanding of the etiology of the clinical findings seen with malrotation.
Normal embryologic development of the alimentary tract
The alimentary tract develops from the embryologic foregut, midgut, and hindgut. Normal rotation takes place around the superior mesenteric artery (SMA) as the axis. It is described by referring to 2 ends of the alimentary canal, the proximal duodenojejunal loop and the distal cecocolic loop, and is usually divided into 3 stages. Both loops make a total of 270° in rotation during normal development. Both loops start in a vertical plane parallel to the SMA and end in a horizontal plane. See the image below.
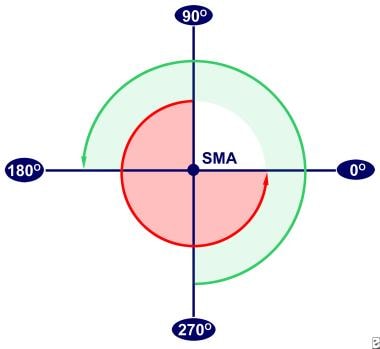 Normal rotation of the intestines during development. The superior mesenteric artery (SMA) is the axis. The duodenojejunal loop (red arrow) begins superior to the SMA, and the cecocolic loop (green arrow) begins inferior to the SMA.
Normal rotation of the intestines during development. The superior mesenteric artery (SMA) is the axis. The duodenojejunal loop (red arrow) begins superior to the SMA, and the cecocolic loop (green arrow) begins inferior to the SMA.
Stage I occurs between 5-10 weeks' gestation. It is the period of physiologic herniation of the bowel into the base of the umbilical cord. The duodenojejunal loop begins superior to the SMA at a 90° position and rotates 180° in a counterclockwise direction. At 180°, the loop is to the anatomical right of the SMA, and by 270°, it is beneath the SMA. The cecocolic loop begins beneath the SMA at 270°. It rotates 90° in a counterclockwise manner and ends at the anatomical left of the SMA at a 0° position. Both loops maintain these positions until the bowel returns to the abdominal cavity. Also during this period, the midgut lengthens along the SMA, and, as rotation continues, a broad pedicle is formed at the base of the mesentery. This broad base protects against midgut volvulus.
Stage II occurs at 10 weeks' gestation, the period when the bowel returns to the abdominal cavity. As it return s, the duodenojejunal loop rotates an additional 90° to end at the anatomical left of the SMA, the 0° position. The cecocolic loop turns 180° more as it reenters the abdominal cavity. This turn places it to the anatomical right of the SMA, a 180° position.
Stage III lasts from 11 weeks' gestation until term. It involves the descent of the cecum to the right lower quadrant and fixation of the mesenteries.
Nonrotation
Arrest in development at stage I results in nonrotation. Subsequently, the duodenojejunal junction does not lie inferior and to the left of the SMA, and the cecum does not lie in the right lower quadrant. The mesentery in turn forms a narrow base as the gut lengthens on the SMA without rotation, and this narrow base is prone to clockwise twisting leading to midgut volvulus. The width of the base of the mesentery is different in each patient, and not every patient with nonrotation develops midgut volvulus.
Incomplete rotation
Stage II arrest results in incomplete rotation and is most likely to result in duodenal obstruction. Typically, peritoneal bands running from the misplaced cecum to the mesentery compress the third portion of the duodenum. Depending on how much rotation was completed prior to arrest, the mesenteric base may be narrow and, again, midgut volvulus can occur. Internal herniations may also occur with incomplete rotation if the duodenojejunal loop does not rotate but the cecocolic loop does rotate. This may trap most of the small bowel in the mesentery of the large bowel, creating a right mesocolic (paraduodenal) hernia.
Incomplete fixation
Potential hernia pouches form when the mesentery of the right and left colon and the duodenum do not become fixed to the retroperitoneum. If the descending mesocolon between the inferior mesenteric vein and the posterior parietal attachment remains unfixed, the small intestine may push out through the unsupported area as it migrates to the left upper quadrant. This creates a left mesocolic hernia with possible entrapment and strangulation of the bowel. If the cecum remains unfixed, volvulus of the terminal ileum, cecum, and proximal ascending colon may occur.
Epidemiology
United States statistics
Intestinal malrotation occurs in between 1 in 200 and 1 in 500 live births. [6, 7] However, most patients with malrotation are asymptomatic, with symptomatic malrotation occurring in only 1 in 6000 live births. [8] Symptoms and diagnosis may occur at any age, with some reports of prenatal diagnosis of intestinal malrotation. [9]
Malrotation may occur as an isolated anomaly or in association with other congenital anomalies; 30-62% of children with malrotation have an associated congenital anomaly. All children with diaphragmatic hernia, gastroschisis, and omphalocele have intestinal malrotation by definition. Additionally, malrotation is seen in approximately 17% of patients with duodenal atresia and 33% of patients with jejunoileal atresia. [10, 11]
Sex- and age-related demographics
Sex
Male predominance is observed in neonatal presentations at a male-to-female ratio of 2:1. No sexual predilection is observed in patients older than 1 year.
Age
Traditional teaching suggests that as many as 40% of patients with malrotation present within the first week of life, 50% in the first month, and 75% in the first year. However, more recent series have shown that malrotation is increasingly identified in adults. A series of 170 patients with intestinal malrotation diagnosed at a single institution between 1992-2009 found that 31% were infants, 21% were aged 1-18 years, and the remaining 48% were adults. [12] Although unusual, there are reports of adults presenting with total small bowel volvulus due to malrotation in adults. [13] A second series found that 42% of patients with a new diagnosis of malrotation were adults. [14] Reports have even documented congenital malrotation presenting during pregnancy. [15, 16, 17]
Prognosis
Prognosis is dependent on the presence of ischemic or necrotic bowel, the amount of bowel resected, and the age of the child. In general, older children have improved morbidity and mortality compared to infants. The presence of midgut volvulus is associated with prolonged hospital length of stay. Long-term prognosis is dependent on how much bowel is resected and the development of short-bowel syndrome.
Morbidity/mortality
Data from recent series reveal that mortality rates in adults and children operated on for intestinal malrotation range from 0-14%. Higher mortality rates are seen in cases with acute onset of midgut volvulus, delayed diagnosis, or the presence of intestinal necrosis. [18, 19, 20, 21, 1, 22] Children with other associated anomalies also have higher overall mortality rates. A report of 25 years' experience demonstrated congenital cardiovascular disease in 27.1% of patients with intestinal malrotation; those patients had a morbidity rate of 61.1% after intestinal malrotation surgery. [23]
Complications
Complications include the following:
-
Short-bowel syndrome: Short-bowel syndrome is the most common complication of midgut volvulus. These patients have longer delays to recovery of bowel motility and function. They are at high risk for malabsorption and can require long-term parenteral nutrition. Furthermore, these patients have more complications from treatment and longer hospital stays than patients with malrotation without volvulus.
-
Infection: Wound infections and sepsis can occur in the immediate postoperative period, requiring extended treatment with intravenous antibiotics. Additionally, central venous catheters have the potential to become infected causing bacteremia and/or sepsis
-
Surgical complications: Postoperative and surgical complications are more likely to occur in those patients with acute symptoms than those with chronic symptoms. [20] One review reported an overall complication rate of 8.7% (14 of 161) following Ladd procedure. [24] Complications reported include adhesive small bowel obstruction in 6% with 5 requiring reoperation (3%), and 1 patient developed recurrent volvulus (1%). A second review showed comparable rates of recurrent volvulus (2%, 1 of 57) and reoperation for adhesive small bowel obstruction (2%, 1 of 57). [25] Other series have reported lower rates of recurrent volvulus, 0.4% in one series of 441 patients, and 0.6% in a series of 159 patients who underwent Ladd's procedure. [26]
-
Persistent GI symptoms: In the same series of 57 patients, 13 had persistent (>6 mo) GI symptoms, including constipation (6), intractable diarrhea (1), abdominal pain (2), vomiting (3), and feeding difficulties (1) following Ladd procedure. [25]
-
Mortality: Death occurs due to peritonitis, late nutritional complications, or catheter-related sepsis. Rates are increased among children younger than one year. Following the Ladd procedure, mortality rates reported in the literature are as low as 2%. [24] However, if more than 75% of the bowel is necrotic, mortality is as high as 65%. [26]
-
Normal rotation of the intestines during development. The superior mesenteric artery (SMA) is the axis. The duodenojejunal loop (red arrow) begins superior to the SMA, and the cecocolic loop (green arrow) begins inferior to the SMA.
-
In this upper GI series with abnormal results, the duodenum does not cross the midline, and the small bowel is present only in the right side of the abdomen.
-
These 2 lower GI series show the cecum (arrows) in the right upper quadrant, indicative of malrotation.
-
This patient had malrotation with midgut volvulus. The gut is darkened in color because of ischemia.





