Practice Essentials
Antiphospholipid antibody syndrome is a systemic autoimmune disorder that can manifest clinically as recurrent thrombosis. [1] The criteria for the diagnosis of pediatric antiphospholipid antibody syndrome have not yet been validated. [2]
Signs and symptoms
Vasospastic or vaso-occlusive events can occur in any organ system in patients with antiphospholipid antibody syndrome. The spectrum of involvement ranges from rapidly progressive to clinically silent and indolent. (See the image below.)
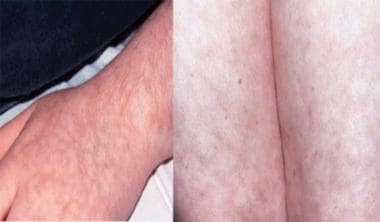 Livedo reticularis of the upper and lower extremities in a 15-year-old adolescent with primary antiphospholipid antibody syndrome. The pattern is lacy, flat, and nonblanching. The purplish hue is from stasis in the small vessel beds.
Livedo reticularis of the upper and lower extremities in a 15-year-old adolescent with primary antiphospholipid antibody syndrome. The pattern is lacy, flat, and nonblanching. The purplish hue is from stasis in the small vessel beds.
Catastrophic antiphospholipid antibody syndrome is a multisystem failure secondary to thrombosis, infarction, or both and is characterized by microangiopathy on histopathologic examination.
See Presentation for more detail.
Diagnosis
Laboratory studies
If the clinical features suggest an antiphospholipid antibody syndrome, a thorough evaluation to detect the presence of at least one of these antibodies is essential. Evaluate the patient for the following:
-
Anticardiolipin
-
Antiphosphatidylethanolamine
-
Antiphosphatidylinositol
-
Antiphosphatidylserine
-
Antiphosphatidylglycerol
-
Antiphosphatidic acid
Evaluate for lupus anticoagulant and anti–beta-2 glycoprotein I antibodies as well. Assessment for abnormalities in phospholipid-dependent tests of coagulation is also recommended.
Imaging studies
Imaging studies are useful to confirm a thrombotic event. For example, in patients with venous thrombotic events (eg, deep vein thrombosis), the following studies have been used:
-
Doppler ultrasonography
-
Venography
-
Ventilation/perfusion scan (to document pulmonary emboli)
In patients with arterial thrombotic events, the following studies have been used:
-
Computed tomography
-
Nuclear imaging
-
Magnetic resonance imaging
-
Doppler ultrasonography
-
Magnetic resonance arteriography
-
Arteriography
See Workup for more detail.
Management
In general, the treatment of antiphospholipid antibody syndrome is individualized according to the patient’s clinical status and history of thrombotic events. Asymptomatic patients who have no risk factors and a negative family history of thrombosis do not require specific treatment. In patients with primary antiphospholipid antibody syndrome with venous thrombosis, the initial treatment consists of heparin followed by warfarin or low molecular weight heparin. In patients with primary antiphospholipid antibody syndrome with arterial thrombosis or infarction, many physicians administer antiplatelet therapy in the absence of other risk factors, but the use of anticoagulants is controversial.
See Treatment and Medication for more detail.
Background
Antiphospholipid (aPL) antibodies have been found in association with clinical symptoms such as deep venous thrombosis, arterial occlusive events (eg, stroke, myocardial infarction), and recurrent fetal loss. They are also associated with vasospastic phenomena such as migraine headache, Raynaud phenomenon, and transient ischemic attack (TIA). [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]
The terminology associated with antiphospholipid antibodies has been fraught with misnomers. Conley and Hartmann observed a prolongation in the prothrombin time (PT) in a series of patients with systemic lupus erythematosus (SLE), which was later termed the lupus anticoagulant (LAC). This term is misleading for the following reasons:
-
The LAC phenomenon can be caused by any number of antibodies to the phospholipid template of the coagulation cascade.
-
These antibodies are frequently found outside the clinical spectrum of SLE.
-
Although these antibodies are responsible for a prolongation of the activated partial thromboplastin time (aPTT) in vitro, they are associated with a hypercoagulable state in vivo.
In the early 1980s, Harris identified anticardiolipin antibodies in a subset of these patients. Since that time, antibodies to phospholipids alone have been determined to be associated with infectious causes more often. In contrast, antibodies to combinations of phospholipids and serum proteins (eg, β 2-glycoprotein I [β 2-GPI] or prothrombin) are more likely associated with the vasculopathic events of antiphospholipid antibody syndrome (APS).
Antiphospholipid antibodies associated with vaso-occlusive events without any underlying disease process is termed the primary antiphospholipid antibody syndrome (PAPS). The presence of antiphospholipid antibodies and a vaso-occlusive event superimposed on an underlying disease, such as SLE or malignancy, is a secondary antiphospholipid antibody syndrome. [14]
Preliminary classification criteria for "definite" antiphospholipid antibody syndrome were proposed in a report from the Eighth International Symposium on Antiphospholipid Antibodies and were published in Arthritis and Rheumatism. [15]
The purpose of the report was to define the essential features of antiphospholipid antibody syndrome in order to facilitate studies of treatment and causation. This definition was intended to encompass the clinical and laboratory features most closely associated with antiphospholipid antibodies in prospective studies based on the strongest experimental evidence. The hope was to use the "cleanest" patient populations for basic research and clinical treatment studies. These criteria were not meant to supplant the physician's clinical judgment in making the diagnosis in any particular patient. Although features such as migraine headache, peripheral vasospasm, and thrombocytopenia were excluded from the published criteria, they were argued to be valid and useful clinical parameters in arriving at the diagnosis of antiphospholipid antibody syndrome in the clinical setting at the Ninth International Symposium on Antiphospholipid Antibodies. [16, 17, 18]
In a consensus conference held at the 11th International Symposium on Antiphospholipid Antibodies, existing evidence on clinical and laboratory features of antiphospholipid antibody syndrome was appraised and amendments to the Sapporo criteria were proposed. The criteria were reiterated to be used for clinical research to define homogenous populations for studies. Therefore, in order to address the needs of clinicians and to expand the data for future research, the discussion included definitions on features of antiphospholipid antibody syndrome that were not included in the updated criteria for use clinically and in research. These were published as the "International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Antibody Syndrome (APS)" in J Thrombosis Haemost. [19]
Updated clinical criteria
Clinical criteria include the following:
-
Vascular thrombosis - One or more clinical episodes of arterial, venous, or small vessel thrombosis in any tissue or organ confirmed by imaging studies, Doppler studies, or histopathology (without significant vessel wall inflammation)
-
Pregnancy morbidity (normal morphology on ultrasonography or direct examination findings)
One or more unexplained fetal deaths at more than 10 weeks’ gestation
One or more premature births at less than 34 weeks’ gestation due to severe preeclampsia, eclampsia, or placental insufficiency
Three or more unexplained consecutive spontaneous abortions at less than 10 weeks’ gestation, excluding maternal anatomic or hormonal abnormalities and paternal and maternal chromosomal causes
In research studies of patient populations that contain more than one type of pregnancy morbidity, investigators are strongly encouraged to stratify subjects according to the 3 groups above.
Updated laboratory criteria
Laboratory criteria include the following:
-
Anticardiolipin (aCL) antibody of the immunoglobulin G (IgG)/immunoglobulin M (IgM) isotype in medium/high titer (>40 IgG phospholipid units [GPL], >40 IgM phospholipid units [MPL], or >99th percentile) on 2 or more occasions at least 12 weeks apart (measured by a b2-GPI–dependent enzyme-linked immunosorbent assay [ELISA]).
-
Lupus anticoagulant on 2 or more occasions at least 12 weeks apart, according to the guidelines set forth by the International Society of Thrombosis and Hemostasis Scientific Subcommittee on Lupus Anticoagulants/Phospholipid-dependent Antibodies. [20]
Prolonged phospholipid-dependent coagulation (eg, aPTT, Kaolin clotting time [KCT], dilute Russell viper venom test, dilute PT)
Failure to correct the prolonged coagulation time by a mix with platelet poor plasma (PPP)
Shortening or correction of the prolonged coagulation time with excess phospholipid
Exclusion of other coagulopathies (eg, factor VIII inhibitor, heparin)
Investigators are strongly advised to classify patients (in research studies) with antiphospholipid antibody syndrome into one of the following categories:
-
I - Patients with more than 1 laboratory criteria (any combination)
-
IIa - Patients with LAC present alone
-
IIb - Patients with aCL antibody present alone
-
IIc - Patients with anti-β 2-glycoprotein-I antibody present alone
A patient must meet at least one clinical and one laboratory criterion for a diagnosis of antiphospholipid antibody syndrome. Classification of antiphospholipid antibody syndrome should be avoided if less than 12 weeks or more than 5 years separate a positive antiphospholipid antibody test and the clinical manifestation. [21]
Patients with antiphospholipid antibody syndrome participating in research studies should be further subgrouped according to the presence or absence of additional risk factors for thrombosis. Patients should not be excluded from APS trials because of coexistent inherited or acquired factors for thrombosis.
Patients with antiphospholipid antibody syndrome participating in research studies who fulfill the revised classification criteria should be classified separately from patients with “features associated with antiphospholipid antibody syndrome” or with “noncriteria features of antiphospholipid antibody syndrome.” These features, which were discussed by the consensus panel but not included in the revised criteria, include the following:
-
Heart valve disease
-
Livedo reticularis
-
Thrombocytopenia
-
Nephropathy
-
Neurological manifestations [22]
-
Immunoglobulin A (IgA) aCL
-
IgA anti-b2GPI
-
Antiphosphatidylserine antibodies
-
Antiphosphatidylethanolamine antibodies
-
Antibodies against prothrombin alone
-
Antibodies to the phosphatidylserine-prothrombin complex
Pathophysiology
The mechanism or mechanisms by which the antiphospholipid antibodies interact with the coagulation cascade to produce clinical events are largely speculative and have not been clearly elucidated. The presence of preexisting or coincident vascular (endothelial) damage along with the identification of an antiphospholipid antibody as requisites for the emergence of a thrombotic complication has been coined the "2-hit" hypothesis. [23, 24, 25, 26, 27, 28, 3, 29, 30, 31, 32, 33, 34, 35, 36, 37]
-
Possible mechanisms by which antiphospholipid antibodies may induce thrombotic events include the following:
Antiphospholipid antibodies may combine with platelet membrane phospholipids, resulting in increased platelet adhesion and aggregation.
Antiphospholipid antibodies may combine with the endothelial cell membrane phospholipids along with b2-GPI and induce endothelial cell damage, impaired prostacyclin production, increased platelet adhesion, and aggregation.
Endothelial cell damage may also result in decreased production of endothelium-derived relaxing factor and, thus, increased vasospasm and ischemia.
Antiphospholipid antibodies can stimulate tissue factor expression by endothelial cells, monocytes, and neutrophils with induction of cellular activation and respiratory burst leading to membrane damage.
In secondary antiphospholipid antibody syndrome, vascular endothelial cell damage has already occurred, enhancing the vascular spasm/occlusion, ischemia/infarction, and reperfusion injury.
b2-GPI may be bound up by antiphospholipid antibodies and (1) prevented from covering up exposed procoagulant inner membrane leaflet phospholipids or (2) blocked from inhibiting platelet prothrombinase activity.
b2-GPI and oxidized low-density lipoprotein (oxLDL) complexes may be bound up by antiphospholipid antibodies, cleared by macrophages, and, thus, promote accelerated development of atherosclerosis in autoimmune patients.
Antiphospholipid antibodies may interfere with the interaction of coagulation protein C and coagulation protein S and, thus, affect the formation of the APC coagulation control complex (activated protein C, protein S, and factor V).
-
Possible mechanisms by which antiphospholipid antibody might be generated include the following:
Autoimmunity may be a factor; a break in tolerance may lead to an "escaped clone."
Closely related to the previously mentioned mechanism is the concept that antiphospholipid antibodies are a response to inner membrane leaflet antigens (ie, phosphoserine) that are exposed in apoptotic blebs on cells not eliminated from the circulation because of an overloaded or defective clearance system.
Antiphospholipid antibodies may also be cross-reactive antibodies induced by exogenous antigens from infectious organisms (eg, viral or bacterial).
Canaud et al studied the molecular pathways involved in the vasculopathy of the antiphospholipid syndrome. The researchers used double immunostaining to evaluate pathway activation in the mammalian target of rapamycin complex (mTORC) and the nature of cell proliferation in the vessels of patients with primary or secondary antiphospholipid syndrome nephropathy. The mTORC pathway is involved in the vascular lesions that occur in patients with antiphospholipid syndrome. According to this study the mTOR inhibitor sirolimus may help prevent vasculopathy in patients with the disease. [38, 39]
Etiology
The causes of antiphospholipid antibody syndrome are unknown (see Pathophysiology).
The association of thrombotic events with preexisting or coincident vascular perturbation is emphasized by the high incidence of antiphospholipid antibody syndrome in patients with the following conditions:
-
Vascular inflammation, vasculitis
Autoimmune disease (eg, systemic lupus erythematosus [SLE] [40] , cryoglobulinemia)
Infectious processes (eg, hepatitis, parvovirus, syphilis)
-
Malignancy (eg, carcinoma, leukemia)
-
Vascular trauma
Postsurgery (eg, cardiac)
Trauma (eg, accidental)
-
Drug-induced state (eg, procainamide, phenytoin, hydralazine, chlorpromazine)
-
Hemodialysis-associated condition (increased antiphospholipid antibody antibodies over time on dialysis)
Cuprophane membrane exposure
Oxidative stress
Epidemiology
United States statistics
Antiphospholipid antibodies are reportedly present in 1-15% of the general population (higher in elderly persons). These antibodies are reportedly present in as many as 70% of patients with SLE; however, the frequency rate of antiphospholipid antibody syndrome (ie, antiphospholipid antibodies plus a clinical event) is far lower.
In patients with SLE, a history of thrombosis was reported in 61% of those with positive test results for LAC, in 52% of those who had positive anticardiolipin antibodies, and 24% of those who had no antiphospholipid antibodies.
International statistics
No major differences have been noted between frequency rates in the United States and frequency rates worldwide. A large multicenter European SLE registry suggests that 3-7% of patients with SLE and antiphospholipid antibodies are at risk for new-onset thrombosis.
Race-, sex-, and age-related demographics
Overall, no specific race predilection has been observed.
The frequency rate of primary antiphospholipid antibody syndrome is skewed by race predilection of risk factors for thrombosis and atherosclerotic disease. The frequency of secondary antiphospholipid antibody syndrome is skewed by race predilection for autoimmune diseases.
In secondary antiphospholipid antibody syndrome, the frequency rate is skewed by the female predominance in autoimmune diseases (eg, SLE) in general. In primary antiphospholipid antibody syndrome, the frequency rate is skewed by the inclusion of pregnancy-related events in the classification schema.
In both antiphospholipid antibody syndrome and primary antiphospholipid antibody syndrome, the frequency rate related to sex is equalized in young patients, especially prior to the onset of puberty.
Antiphospholipid antibody syndrome has been described in patients of all ages. The prenatal, perinatal, and neonatal periods can be affected. The onset of disease has been reported in children as young as 8 months. [13]
Prognosis
The long-term prognosis varies and depends on the tissue damage incurred and the organ system or systems affected. Clinical manifestations that are associated with a worse prognosis include the following [41, 42, 43] :
-
Pulmonary hypertension
-
Neurologic involvement (eg, CNS involvement, transverse myelopathy)
-
Myocardial ischemia
-
Nephropathy
-
Gangrene of the extremities
-
Catastrophic antiphospholipid antibody syndrome
Morbidity/mortality
Mortality and morbidity are related to clinical manifestations. An increased incidence of the following is seen in young individuals:
-
Cerebrovascular accident (CVA), stroke
-
Myocardial infarction
-
Endocarditis (may lead to valvular replacement)
-
Pulmonary emboli (may lead to pulmonary hypertension)
-
Deep vein thrombosis (DVT)
-
Fetal loss from second trimester to the perinatal period, including intrauterine growth retardation (IUGR), prematurity, and symptoms of toxemia
-
Catastrophic antiphospholipid antibody syndrome (multisystem failure secondary to thrombosis, infarction, or both may lead to death in 50% of cases)
Complications
Hemorrhage may occur as a result of overaggressive therapy.
Rethrombosis may occur as a result of inadequate therapy.
Catastrophic antiphospholipid antibody syndrome can lead to death (50% mortality rate).
Patient Education
Lifestyle counseling is indicated to educate patients and their families about the risk factors that are known to complicate the prognosis of patients with antiphospholipid antibody syndrome.
Dietary manipulation is recommended to prevent obesity, hyperlipidemia, and hypertension, starting at a young age, especially in patients with a family history of these problems.
Dietary manipulation is recommended to decrease consumption of methionine-containing foods that might increase homocysteine levels in patients carrying mutations of the gene that encodes for methylene tetrahydrofolate reductase mutation (MTHFR). Folate deficiencies need to be identified and corrected in these patients to control homocysteine levels.
Counsel adolescents about the potential risks of smoking tobacco in this setting. Provide smoking cessation programs for patients who already have started smoking.
In patients with a secondary antiphospholipid antibody syndrome, encourage compliance with medications for control of underlying disease processes, such as vasculitis and systemic lupus erythematosus (SLE).
Dietary counseling is indicated for patients on oral anticoagulants, such as the following recommendations:
-
Maintenance of a consistent diet of foods containing vitamin K
-
Avoidance of foods and herbs with anticoagulant properties
Counsel patients regarding the risks of oral contraceptive use and the need for alternative methods of contraception.
Current information for patients and their families can be obtained from the excellent Web sites from the St. Thomas Hospital in London (Hughes Syndrome, Hughes Syndrome Foundation).
For excellent patient education resources, visit eMedicineHealth's Brain and Nervous System Center. Also, see eMedicineHealth's patient education articles Blood Clot in the Legs and Stroke.
-
Palmar livedo reticularis associated with antiphospholipid antibody syndrome may range from a lacy, flat, reticulated pattern to a more confluent, nonblanching, slightly raised rash (secondary to extravasation of RBCs and plasma).
-
Livedo reticularis of the upper and lower extremities in a 15-year-old adolescent with primary antiphospholipid antibody syndrome. The pattern is lacy, flat, and nonblanching. The purplish hue is from stasis in the small vessel beds.
-
Muddy discoloration and mild diffuse swelling of the fingers observed as part of the Raynaud phenomenon, which is associated with antiphospholipid antibody syndrome. At room temperature, this patient still has decreased capillary refill and cold fingers despite treatment with pentoxifylline. The discoloration extends proximally onto the palms and turns blue-purple when exposed to cold.
-
Linear splinter hemorrhages are found under the nails of fingers and toes. These may be solitary or multiple and appear intermittently.
-
One set of suggested algorithms for the workup and treatment of patients with antiphospholipid antibody syndrome. This should not be considered dogmatic because laboratory evaluation is not standardized and treatment remains empiric and controversial. Laboratory testing is not recommended in healthy asymptomatic individuals with no risk factors and a negative family history.
-
Occlusion of the right middle cerebral artery in a 3-year-old child with severe headache and hemiparesis associated with anticardiolipin antibodies.
-
Organizing thrombus in an aortic valve in a patient with positive test results for antiphospholipid antibody and lupus anticoagulant who has systemic lupus erythematosus (SLE) and recurrent thrombotic events. The authors acknowledge the help of Hannes Vogel, MD, in preparing this image.
-
High-power degenerating aortic valve in a patient who has positive test results for antiphospholipid antibody and lupus anticoagulant and who has systemic lupus erythematosus (SLE) and recurrent thrombotic events. The authors acknowledge the help of Hannes Vogel, MD, in preparing this image.
-
Trichrome stain of a thrombus in the intestinal serosa in a patient who has positive test results for antiphospholipid antibody and lupus anticoagulant and who has systemic lupus erythematosus (SLE) and catastrophic antiphospholipid antibody syndrome (CAPS). The authors acknowledge the help of Hannes Vogel, MD, in preparing this image.
-
Antiphospholipid antibody syndrome in a patient with positive test results for antiphospholipid antibody and lupus anticoagulant who has systemic lupus erythematosus (SLE), World Health Organization (WHO) class IV lupus nephritis, and acute renal failure. Top: Thrombosed kidney vessels (periodic acid-Schiff [PAS], original magnification X40). Bottom: Thrombosed kidney vessels (PAS, original magnification X20). Lumen is filled with eosinophilic fibrin with overlying injured endothelial cells. The authors acknowledge the help of Karen W. Eldin, MD, in preparing this image.
-
Antiphospholipid antibody syndrome in a patient with positive test results for antiphospholipid antibody and lupus anticoagulant who has systemic lupus erythematosus (SLE), World Health Organization (WHO) class IV lupus nephritis, and acute renal failure. Top: Thrombosed kidney vessel (hematoxylin and eosin [H&E] stain, original magnification X20). Lumen is occluded with fibrin. A perivascular stromal reaction with degenerating inflammatory cells is observed. Bottom: Thrombosed kidney vessel (H&E stain, original magnification X20). Lumen is occluded with fibrin. The authors acknowledge the help of Karen W. Eldin, MD, in preparing this image.
-
Antiphospholipid antibody syndrome in a patient with positive test results for antiphospholipid antibody and lupus anticoagulant who has systemic lupus erythematosus (SLE), World Health Organization (WHO) class IV lupus nephritis, and acute renal failure. Thrombosed kidney vessel with recanalization (arrows) (Jones stain, original magnification X20). Architectural distortion in the surrounding stroma is observed. The authors acknowledge the help of Karen W. Eldin, MD, in preparing this image.
-
Antiphospholipid antibody syndrome in a patient with positive test results for antiphospholipid antibody and lupus anticoagulant who has systemic lupus erythematosus (SLE) and thrombocytopenia. Livedo reticularis of the upper extremities, which developed as petechiae in the classic lacy, reticular pattern, is observed.
-
Livedo reticularis of the upper extremities, which developed as petechiae in the classic lacy, reticular pattern and evolved as a confluent, nonblanching, slightly raised purpuric rash in the same reticular pattern.
-
Digital infarctions in a patient with systemic lupus erythematosus with antiphospholipid syndrome (APS) and long-standing Raynaud symptoms. Multiple and repeated digital infarctions are depicted, resulting in ulcerations and scarring. Scars and hyperpigmentation are also seen on the palmer aspect of hands and fingers.
-
A patient with multisystem small vessel coagulopathy (microangiopathy) but no known underlying disease process. Extensive involvement of all digits is noted, some with distal infarction and dry gangrene, others healing with residual eschar (and undermining epithelialization), and some with re-epithelialization and scarring. Healed superficial epidermal damage and desquamation is also present.
-
A patient with multisystem small vessel coagulopathy (microangiopathy) but no known underlying disease process. Eschar is still present on first digit bilaterally. More superficial lesions are shown here, with evolution and healing of lesions on all other toes.
-
CAPS, Bone Infarction - MRI (High Resolution Proton Density and STIR images) and Nuclear Bone Scan - Patient with multisystem small vessel coagulopathy (microangiopathy) but no known underlying disease process. MRI shows multiple infarctions in the distal tibia, tarsal bones and metatarsal bones (extensive bone marrow edema and increased T1 with fat saturation signal in the calcaneus bones). Flow and early blood pool images of technetium 99m bone scan show increase in activity in both heel regions with focal areas of decreased activity in the center of each calcaneus.
-
A patient with multisystem small vessel coagulopathy (microangiopathy) but no known underlying disease process. The technetium 99m bone scan reveals irregular multifocal areas of tracer accumulation within the left ventricle of the heart suggestive of myocardial infarction and altered calcium deposition. Irregular cutaneous and subcutaneous uptake is noted in multiple areas of the torso and upper arms (as well as in the upper thighs). High-resolution CT scanning of the chest reveals extensive calcification involving the myocardium, the mitral and tricuspid valve annuli, the aortic valve annulus, the proximal right coronary artery, and the left main coronary artery.






