Background
Our understanding of the anatomy, physiology, biochemistry, and molecular biology of neonatal breathing has increased in the past 20 years. [1, 2, 3, 4, 5, 6] For instance, data are elucidating the genes involved in the embryonic development of central respiratory centers and their neural networks. [7, 8, 9] The central respiratory generator is essential for fetal breathing movements. It appears early in pregnancy and importantly contributes to pulmonary development. [10]
In the fetus, breathing is intermittent and occurs during the low-voltage electrocortical state (analogous to rapid eye movement [REM] sleep) and becomes continuous immediately after birth. The regulatory neurologic mechanisms that cause the transition from intermittent fetal breathing to continuous neonatal breathing are incompletely appreciated. [11, 12]
After birth, apnea of prematurity (AOP) is a major concern for caregivers in intensive care nurseries. The magnitude of this problem resulted in the National Institutes of Child Health and Human Development (NICHD) convening a workshop on apnea of prematurity. Summary Proceedings from the Apnea-of-Prematurity Group have been published.
The NICHD review group emphasized the following conclusions [13] :
-
No consensus has been reached regarding the definition, diagnosis, or treatment of apnea of prematurity.
-
Systematic research has not been conducted to investigate the value of different interventions for apnea of prematurity.
-
Available technology is not routinely used to document real-time events associated with apnea.
-
The time required to demonstrate an improvement in apnea of prematurity with a specific treatment has not been established.
-
The observational period needed after therapy for apnea of prematurity is unknown, and an appropriate duration of surveillance off therapy is needed to reasonably prevent acute life-threatening events.
-
Important confounding conditions that influence the occurrence of apnea of prematurity are poorly recognized and/or integrated into care.
-
The relationship between gastroesophageal reflux (GER) and apnea of prematurity requires additional investigation because current knowledge suggests an infrequent association.
-
Improved characterization of the effects of apnea of prematurity on neurodevelopment during infancy and childhood is needed.
-
Other confounders associated with brain injury in preterm infants are difficult to separate from apnea of prematurity as meaningful causes of abnormal child development.
The NICHD review group also made recommendations about what issues associated with apnea of prematurity that need urgent attention, what research methods might be best for future studies, what outcomes are essential to our understanding of apnea of prematurity, and what ethical principles should govern future investigations of apnea of prematurity.
Given this discussion from the NICHD review group, the present article provides state-of-the-art information regarding what is and what is not known about apnea of prematurity.
Definitions
Apnea is defined as the cessation of breathing for more than 20 seconds or the cessation of breathing for less than 20 seconds if it is accompanied by bradycardia or oxygen (O2) desaturation. [14] Note the following:
-
Bradycardia in a premature neonate is considered clinically significant when the heart rate slows by least 30 bpm from the resting heart rate.
-
An O2 saturation level of less than 85% is considered pathologic in this age group, as is a decrease in O2 saturation should it persist for 5 seconds or longer.
These definitions represent clinically significant changes in apnea, bradycardia, and O2 saturation changes and rarely occur in healthy preterm neonates older than 36 weeks after conception.
Apnea is classified as central, obstructive, or mixed. Note the following:
-
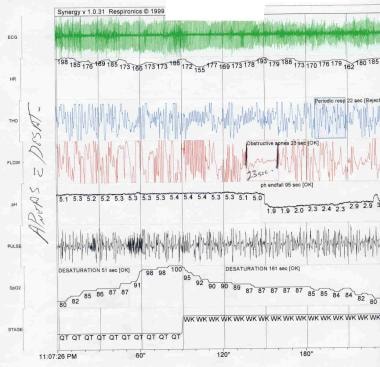
Central apnea is defined as complete cessation of respiration, which can be differentiated from obstructive apnea through a pneumogram, with cessation of airflow and respiratory effort (see the image below).
-
Obstructive apnea is the cessation of airflow in the presence of continued respiratory effort.
-
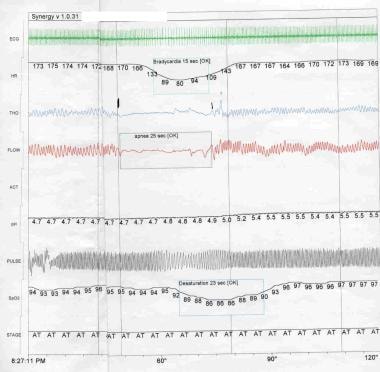
Mixed apnea contains elements of both central and obstructive apnea (see the image below), either within the same apneic pause or at different times during a period of respiratory recording.
 Apnea of Prematurity. Polysomnogram. Mixed apnea contains elements of both central and obstructive apnea. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement; SpO2 = peripheral oxygen saturation (%); STAGE = sleep stage, where AT = active sleep.
Apnea of Prematurity. Polysomnogram. Mixed apnea contains elements of both central and obstructive apnea. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement; SpO2 = peripheral oxygen saturation (%); STAGE = sleep stage, where AT = active sleep.
Apnea of infancy
Apnea of infancy (AOI) occurs when apnea persists in a neonate older than 37 weeks after conception. The physiologic aspects of apnea of prematurity and AOI coincide, though further studies are needed to determine their exact relationship.
Periodic breathing
Periodic breathing is defined as periods of regular respiration for as long as 20 seconds followed by apneic periods of 10 seconds or less that occur at least 3 times in succession (see the image below).
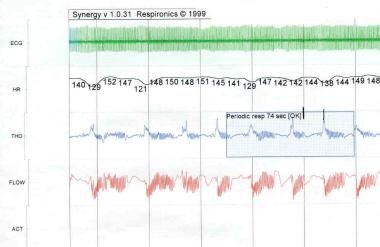 Apnea of Prematurity. Polysomnogram. Periodic breathing is defined as periods of regular respiration for as long as 20 seconds followed by apneic periods no longer than 10 seconds that occur at least 3 times in succession. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement.
Apnea of Prematurity. Polysomnogram. Periodic breathing is defined as periods of regular respiration for as long as 20 seconds followed by apneic periods no longer than 10 seconds that occur at least 3 times in succession. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement.
Periodic breathing may be observed for 2-6% of the breathing time in healthy term neonates and as much as 25% of the breathing time in preterm neonates. The occurrence of periodic breathing is directly proportional to the degree of prematurity.
Kelly and coworkers observed periodic breathing in 78% of neonates examined at 0-2 weeks of age. [15] The incidence substantially declined to 29% at the postconceptual ages of 39-52 weeks.
Periodic breathing typically does not occur in neonates during their first 2 days of life.
Periodic breathing most frequently occurs during active sleep, but it can also happen when neonates are awake or quietly sleeping. This pattern, commonly observed in patients at high altitudes, is eliminated with supplemental oxygenation and/or with the use of continuous positive airway pressure (CPAP). Because the prognosis is excellent and because the infant is not compromised, no treatment is usually required.
Pathophysiology
Central respiratory regulation
Immaturity and/or depression of the central respiratory drive to the muscles of respiration have been accepted as key factors in the pathogenesis of apnea of prematurity. [1] Vulnerability of the ventral surface of the medulla and adjacent areas in the brainstem to inhibitory mechanisms is the likely explanation for why apneic episodes occur in prematurely born infants. This vulnerability involves diverse clinicopathologic events. [4, 16] Inhibitory events that affect the central respiratory generator and initiate apnea include hypoxia, hyperthermia and adenosine secretion. [17] Studies of preterm infants and in animals (especially genetically altered mice) have enhanced our understanding of the molecular and biochemical events leading to maturation of the central respiratory generator in preterm infants. [2, 3, 18, 19]
Using noninvasive techniques, Henderson-Smart and coworkers documented that brainstem conduction times of auditory-evoked responses were longer in infants with apnea than in matched premature infants without apnea. [20] This study elucidated apnea in preterm infants by indirectly showing that infants with apnea had greater-than-expected immaturity of brainstem function, which was based on postconceptional age. This finding supports the concept than an immature brainstem eventually develops control of breathing as dendritic spines and synaptic connections mature. An observation that emphasizes the importance of the central respiratory generator is the finding of increased apnea among preterm infants with bilirubin-encephalopathy diagnosed by using abnormal auditory brainstem-evoked responses. [21]
The absence of respiratory muscle activity during central apnea unequivocally implicates depression of respiratory center output. In support of this concept, Gauda and associates (1989) documented reduced electromyographic activity in the diaphragm during spontaneous obstructed inspiratory efforts. [22] Such efforts characterize combined central and obstructive apnea. [23] Therefore, episodes of both central and mixed apneic share an element of decreased respiratory center output to the respiratory muscles.
Sleep state and apnea
Apnea during infancy occurs most frequently during active or REM sleep. [24, 25] Apnea occurs relatively infrequently during quiet sleep, when respiration is characteristically regular, with little breath-to-breath variation in tidal volume and respiratory rate. However, periodic breathing may predominantly occur during non-REM sleep. During active sleep, respiration is mostly paradoxical due to spinal motoneural inhibition of the activity of intercostal muscles. [26]
In extremely preterm infants, the paucity of quiet sleep, together with an extremely compliant rib cage, makes paradoxical chest-wall movements almost a constant phenomenon. Paradoxical chest movement may predispose the baby to apnea by decreasing functional residual capacity (FRC) and limiting oxygenation. [27]
Chemoreceptors and mechanoreceptors
Complex relationships exist between respiratory control; several sites of central chemosensitivity to carbon dioxide (CO2) during sleep; and various neuromechanical factors originating in the lungs, chest wall, and upper airway that modify respiratory function during sleep. [28]
Responses of chemoreceptors in preterm and term neonates have been investigated. [29, 30, 31] The response to elevated CO2 concentrations was blunted in prematurely born infants. This diminished response may partly be due to decreased central chemosensitivity or mechanical factors that prevent an adequate ventilatory response. [32, 33]
The slope of the response curve for CO2 is decreased for preterm infants who have apnea. [34] However, a cause-and-effect relationship between decreased CO2 responsiveness and apnea of prematurity has not been clearly established. Administration of CO2 ameliorates periodic breathing, but inhalation of CO2 is not a therapeutic option for human infants.
For many years, scientists have known that preterm infants respond to a decrease in inspired O2 concentration with a transient increase in ventilatory response, followed by return to baseline or even depression of ventilation. [35] This response to low O2 in infants appears to result from initial stimulation of peripheral chemoreceptors then overriding depression of the respiratory center as a result of hypoxemia. [36] Consistent with these findings is the observation that a progressive decrease in inspired O2 concentrations causes a significant flattening of CO2 responsiveness in preterm infants. [37]
This unstable response to low inspired O2 levels may play an important role in the origin of neonatal apnea. It offers a physiologic rationale for the decrease in incidence of apnea observed when a slightly increased concentration of inspired O2 is administered to infants with apnea. [38]
The Hering-Breuer reflex also plays an important role in modulating respiratory timing in human neonates. Pulmonary stretch receptors send an afferent neural input to brain and mediate the Hering-Breuer reflex by means of the vagus nerve. Thereafter, they inhibit inspiration, prolong expiration, or both, while increasing lung volume. [27] Active shortening of expiratory duration with decreased lung volume may provide a breathing strategy for preserving FRC in a neonate with a highly compliant chest wall.
Upper airway obstruction substantially contributes to apneic episodes in preterm infants, and upper airway muscles show preferential reflex activation in response to airway obstruction in infants. [39]
Gerhardt and Bancalari compared the ability of preterm infants with and those without apnea to respond to end-expiratory airway occlusion. [34, 40] Prolongation of the occluded inspiratory effort was significantly prolonged in the group without apnea. This finding suggested that this group had a relatively mature respiratory reflex response that improved their ability to respond to airway obstruction.
In premature infants, complex changes in pulmonary mechanics and ventilatory timing accompany apnea. [41] Before apnea occurs, total pulmonary resistance may increase in association with a decrease in tidal volume and prolongation of the expiratory time. Such changes have been noted before episodes of mixed, obstructive, and central apnea.
In 1982, Waggener and coworkers showed that a diminution in respiratory drive precedes apnea, a finding reminiscent of the cyclic alterations in respiratory drive. [42] After apnea resolves and respiration resumes, the respiratory drive in premature infants initially increases, possibly because of a cumulative effect of hypoxia and hypercapnia. Total pulmonary and supraglottic resistance also increases, perhaps in response to a decrease in lung volume and collapse of the upper airway when respiratory drive declines during the apnea.
Of note, within 2 or 3 breaths after apnea, pulmonary resistance and respiratory drive is restored to normal pre-apnea values in premature infants. Therefore, the neural systems that restore respiratory homeostasis appear to be capable of mounting an adequate response, even in premature infants with apnea.
Upper airway instability and muscles of the chest wall
Premature infants have pharyngeal or laryngeal obstruction during spontaneous apnea.
Thach (1983) proposed a model in which the negative luminal pressures generated during inspiration in the upper airway predispose a compliant pharynx to collapse. [43]
Many muscles of the upper airway, especially the genioglossus muscles, have been widely implicated in mixed and obstructive apnea affecting both infants and adults. Carlo, Martin, and Difiore compared the activity of the genioglossus muscles with that of the diaphragm in response to hypercapnic stimulation. [44] In preterm infants, genioglossus activation was delayed for about one minute after CO2 rebreathing was begun, and it occurred only after a CO2 threshold of approximately 45 mm Hg was reached.
In neonates inspiratory time is often modestly prolonged when end-expiratory airway occlusion prevents lung inflation. As indicated earlier, this effect is a manifestation of the Hering-Breuer reflex.
Studies in animals demonstrated that this vagally mediated inhibition of normal lung inflation has more influence on the upper airway muscles than on the diaphragm. [45]
Upper airway reflexes
The upper airway contains many sensory nerve endings that may respond to various chemical and mechanical stimuli. Sensory input from these upper airway receptors travels to the CNS by means of cranial nerves V, VI, IX, X, XI, and XII. They may strongly affect respiratory rate and rhythm, heart rate, and vascular resistance. [46]
The chemoreceptor drive may augment the ability of the upper airway muscles to respond to increasing negative pressure, whereas input from pulmonary stretch receptors inhibits it. [47]
Swallowing during the respiratory pause is unique to apnea and does not occur during periodic breathing. [48]
Effects of adenosine
Adenosine and its analogs cause respiratory depression. [49] Adenosine antagonism is proposed as a mechanism to explain the therapeutic effect of aminophylline. [50]
Gastroesophageal reflux
GER and apnea are common in preterm infants. Because they often coexist, a lively and ongoing debate persists among healthcare professionals about the role of GER in apnea of prematurity.
An extensive literature review was undertaken to justify arguments about the role of GER in apnea of prematurity. Monitoring studies demonstrated that, when a relationship between reflux and apnea is observed, apnea may precede rather than follow reflux. [51, 52] During an apneic episode, loss of respiratory neural output may be accompanied by a decrease in lower esophageal tone, and GER occurs.
This phenomenon is supported by data from a newborn piglet model, which showed that hypoxia and apnea were accompanied by a reduction in lower esophageal sphincter pressure, which was a predisposing factor for GER. [53]
GER and apnea are also discussed in Differentials.
Etiology
The physiology related to apnea of prematurity is reviewed in Pathophysiology. Aspects of causation are briefly reemphasized here.
A premature neonate in whom all other causes of apnea have been excluded may be considered to have apnea of prematurity. Although the etiology of apnea of prematurity is not fully understood, several mechanisms have been proposed to explain this condition, including those described below.
-
Apnea of prematurity is the clinical phenomenon associated with incompletely organized and interconnected respiratory neurons in the brainstem and their response to a multitude of afferent stimuli. Therefore, the abnormal control of breathing seen in apnea of prematurity represents neuronal immaturity of the brain. (For an excellent review of this topic, see the article by Darnall et al. [1] )
-
In a premature neonate, protective respiratory reflex activity is decreased, and Hering-Breuer reflex activity is increased.
-
Dopaminergic receptors may have a role in inhibiting the responses of peripheral chemoreceptor and hypoxia-elicited central neural mechanisms. Evidence from studies of neonatal animals indicates that endogenous endorphin production may depress the central respiratory drive. Although endogenous opiates may modulate the ventilatory response to hypoxia in newborn animals, a competitive opiate receptor antagonist (naloxone) has no therapeutic role in apnea of prematurity.
-
Negative luminal pressures are generated during inspiration, and the compliant pharynx of the premature neonate is predisposed to collapse. Failure of genioglossus activation is most widely implicated in mixed and obstructive apnea among infants and adults.
-
The ability of medullary chemoreceptors to sense elevated CO2 levels is impaired. Therefore, an absent, small, or delayed response of the upper airway muscles to hypercapnia might cause upper airway instability when a linear increase in chest-wall activity also occurs. This impairment may predispose the infant to obstructed inspiration after a period of central apnea.
-
Another important factor to consider is the excitation of chemoreceptors in the larynx due to acid reflux. Laryngeal receptors send afferent fibers to the medulla and can elicit apnea when stimulated.
-
Swallowing during a respiratory pause is unique to apnea and does not occur during periodic breathing. Accumulation of saliva in the pharynx could hypothetically prolong apnea by means of a chemoreflex mechanism.
-
Some practicing neonatologists believe that gastroesophageal reflux (GER) is associated with recurrent apnea and have, therefore, treated preterm neonates with antacid and/or antireflux drugs. However, this assumption has been vigorously challenged.
-
Booth suggested that apneic episodes were reduced when esophagitis resolved because apnea clinically improved 1 or 2 days after the start of antireflux therapy. Therefore, neonatologists have treated xanthine-resistant apnea with H2 blockers, metoclopramide, thickened formula, and/or upright positioning during feeding.
-
No controlled trials have demonstrated that antireflux drugs are effective in preventing apnea; on the contrary, data suggest that it may be harmful. [54]
-
Findings from several studies have not demonstrated a relationship between episodes of apnea and episodes of acid reflux into the esophagus (see Pathophysiology and Differentials).
-
Menon, Schefft, and Thach observed that regurgitation of formula into the pharynx after feeding was associated with an increased incidence of apnea in premature infants. [55] As stated above, gastric fluids can possibly activate laryngeal chemoreflexes, leading to apnea.
-
Although well-designed, controlled clinical trials are few, scientists often say that aminophylline exacerbates reflux in infants with apnea. The relationship of GER to methylxanthines is based on the literature about asthma, and limited studies in neonatal only suggest its occurrence. [56] Some authors have not related the use of methylxanthine to severe GER disease. [57]
Epidemiology
United States data
Although not always apparent, apnea of prematurity is the most common problem in premature neonates. Approximately 70% of babies born before 34 weeks of gestation have clinically significant apnea, bradycardia, or O2 desaturation during their hospital stay. The more immature the infant, the higher his or her risk of apnea of prematurity. Apnea may occur during the postnatal period in 25% of neonates who weighed less than 2500 g at birth and in 84% of neonates who weigh less than 1000 g.
Carlo and Barrington showed that apnea may begin on the first day of life in neonates without respiratory distress syndrome. [58, 59] However, apnea of prematurity is always a diagnosis of exclusion. Many diseases manifest with apnea on the day of birth; examples are intrapartum magnesium exposure, systemic infections or the fetal inflammatory response syndrome, pneumonia, intracranial pathology, seizures, hypoglycemia, and other metabolic disturbances.
Approximately 50% or more of surviving infants who weighed less than 1500 g at birth have episodes of apnea that must be managed with pharmacologic intervention or ventilatory support. Mixed apnea accounts for about 50% of all cases of apnea in premature neonates; about 40% are central apneas, and 10% are obstructive apneas. [60] These percentages vary in different reports. In 50% of all apneic episodes, an obstructive component precedes or follows central apnea, which leads to mixed apnea.
International data
To the authors' knowledge, no investigators have compared the incidence of apnea of prematurity in the United States with those of other countries.
Race-, sex-, and age-related demographics
The authors know of no systematic, prospective clinical study that has been conducted to evaluate the role of a person's race/ethnic background or sex on the incidence of apnea of prematurity.
A young gestational age at birth is associated with an increased incidence of apnea of prematurity. The age at which apnea of prematurity resolves depends on several factors. The mean time for severe apnea of prematurity to resolve is approximately 43 weeks after conception, but a prolonged duration of risk is not uncommon. [14]
In one report, about 6-22% of babies with a very low birth weight had apnea at term. [61] Approximately 91% of premature neonates had apnea of longer than 12 seconds at the time of hospital discharge. Of these babies, 31% also had bradycardia, and 6.5% required prolonged hospitalization because of the severity of their apnea and bradycardia.
These findings show that apnea of prematurity does not resolve at term in many low birth weight infants and that it may persist for some time after hospital discharge.
Prognosis
Regarding the natural history of apnea in infants born prematurely, the frequencies of all types of apnea gradually decreases during the first months of postnatal life. However, in some infants, apnea may persist until the age of 44 weeks after conception.
Morbidity/mortality
Butcher-Puech and coworkers found that infants in whom obstructive apnea exceeded 20 seconds had an increased incidence of intraventricular hemorrhage, hydrocephalus, prolonged mechanical ventilation, and abnormal neurologic development after their first year of life. [62]
In 1985, Perlman and Volpe described a decrease in the cerebral blood flow velocity that accompanies severe bradycardia (heart rate < 80 bpm). [63] Infants with clinically significant apnea of prematurity do not perform as well as prematurely born infants without recurrent apneas during neurodevelopmental follow-up testing. [64, 65]
Complications
Infants born prematurely are at increased risk for apnea and bradycardia after undergoing general anesthesia or sedation with ketamine, regardless of their history of apnea. Because of this increased risk, defer elective surgery, if possible, until approximately 52-60 weeks after conception to allow the infant's respiratory control mechanism to mature.
Patient Education
Family members and others involved in the care of an infant with apnea of prematurity should be well trained in cardiopulmonary resuscitation (CPR).
Many of the pitfalls of home monitoring can be avoided by providing 24-hour telephone access (the ideal level of service) to a designated physician or nurse who is involved in the infant's care. In addition to this access, families should receive frequent, regularly scheduled telephone calls from healthcare providers, as well as home visits by a nurse or respiratory technician or follow-up appointments in a clinic familiar with this field of care.
-
Apnea of Prematurity. Central apnea is defined as the cessation of both airflow and respiratory effort. ECG = electrocardiogram; HR = heart rate; THO = thoracic impedance; FLOW = air flow; ACT = ; SpO2 = peripheral oxygen saturation; STAGE = sleep stage.
-
Apnea of Prematurity. Polysomnogram. Mixed apnea contains elements of both central and obstructive apnea. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement; SpO2 = peripheral oxygen saturation (%); STAGE = sleep stage, where AT = active sleep.
-
Apnea of Prematurity. Polysomnogram. Periodic breathing is defined as periods of regular respiration for as long as 20 seconds followed by apneic periods no longer than 10 seconds that occur at least 3 times in succession. ECG = electrocardiogram; HR = heart rate (bpm); THO = thoracic movement; FLOW = flow the from nose and mouth; ACT = gross body movement.