Background
Hymenolepiasis is the most common intestinal tapeworm infection of humans caused by worm of family cestoda, genus Hymenolepis and species nana. This infection does not require an intermediate host and infection can occur directly from one infected person to another by fecal-oral transmission. H nana is commonly known as the dwarf tapeworm. Another less frequent zoonotic intestinal tapeworm infection is caused by H diminuta, commonly known as the rat tapeworm, in which humans are incidental hosts. [1]
Pathophysiology
The life cycles consist of adult (tapeworm) stages in the small bowel of humans and rodents, and also larval tissue stages in insects (cysticercoid). In addition, the cysticercoid stages of H nana can also invade and develop in the human intestine thus is capable of completing its entire life cycle in the human host. H nana can also be transmitted through autoinfection without having to pass through the insect host.
Life cycle and mode of transmission of H nana: [1]
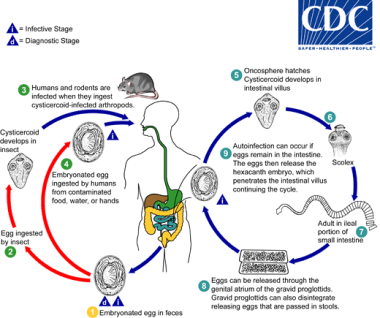 Life cycle of Hymenolepis nana. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
Life cycle of Hymenolepis nana. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
Eggs of Hymenolepis nana are immediately infective when passed with the stool and cannot survive more than 10 days in the external environment (1). When eggs are ingested by an arthropod intermediate host (2) (various species of beetles and fleas may serve as intermediate hosts), they develop into cysticercoids, which can infect humans or rodents upon ingestion (3) and develop into adults in the small intestine. A morphologically identical variant, H. nana var. fraterna, infects rodents and uses arthropods as intermediate hosts. When eggs are ingested (4) (in contaminated food or water or from hands contaminated with feces), the oncospheres contained in the eggs are released. The oncospheres (hexacanth larvae) penetrate the intestinal villus and develop into cysticercoid larvae (5). Upon rupture of the villus, the cysticercoids return to the intestinal lumen, evaginate their scoleces (6), attach to the intestinal mucosa and develop into adults that reside in the ileal portion ofthesmallintestine producing gravid proglottids (7). Eggs are passed in the stool when released from proglottids through its genital atrium or when proglottids disintegrate in the small intestine (8). An alternate mode of infection consists of internal autoinfection, where the eggs release their hexacanth embryo, which penetrates the villus continuing the infective cycle without passage through the external environment (9). The life span of adult worms is 4 to 6 weeks, but internal autoinfection allows the infection to persist for years. [1]
Life cycle and mode of transmission of H. dimunita: [1]
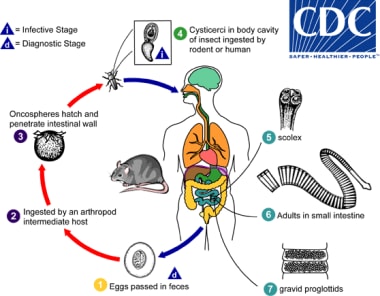 Life cycle of Hymenolepis dimunita. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
Life cycle of Hymenolepis dimunita. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
Eggs of Hymenolepis diminuta are passed out in the feces of the infected definitive host (rodents, man) (1). The mature eggs are ingested by an intermediate host (various arthropod adults or larvae) (2), and oncospheres are released from the eggs and penetrate the intestinal wall of the host (3), which develop into cysticercoid larvae. Species from the genus Tribolium are common intermediate hosts for H. diminuta. The cysticercoid larvae persist through the arthropod's morphogenesis to adulthood. H. diminuta infection is acquired by the mammalian host after ingestion of an intermediate host carrying the cysticercoid larvae (4). Humans can be accidentally infected through the ingestion of insects in precooked cereals, or other food items, and directly from the environment (e.g., oral exploration of the environment by children). After ingestion, the tissue of the infected arthropod is digested releasing the cysticercoid larvae in the stomach and small intestine. Eversion of thescoleces (5) occurs shortly after the cysticercoid larvae are released. Using the four suckers on the scolex, the parasite attaches to the small intestine wall. Maturation of the parasites occurs within 20 days and the adult worms can reach an average of 30 cm in length (6). Eggs are released in the small intestine from gravid proglottids (7) that disintegrate after breaking off from the adult worms. The eggs are expelled to the environment in the mammalian host's feces (1).
Epidemiology
United States data
Infection is most common in the Southeast (1% of school children in one study) and among institutionalized children. Among the 216,275 stool specimens tested in the year of 1987, 0.4% tested positive for H nana. [2] In a study done in western states of Colorado, Utah, New Mexico and Montana; only 1 out of 795 stool samples tested in spring season were positive for H. nana (0.1%), as compared to 33% positive stools for Blastocystis hominis. [3]
The infection rates were found to be higher among Southeast Asian refugees (4.7% and 5.2%) in the Unites States in two different studies. [4, 5] In a large study of internationally adopted children in the USA, H nana was found in the stools of 1% of the 1042 children screened. [6]
International data
Infection is most common in children aged 4-10 years, in dry, warm regions of the developing world. H nana infection affects millions of people, primarily children, worldwide. Estimated rates of infection in various regions range from 0.1-58%. It is estimated to have 50-75 million carriers of H. nana with 5 to 25% prevalence in children worldwide, which can be as high as 50% in children between 1-4 years of age. [7] Regions with high reported infection rates include Sicily (46%), Argentina (34% of school children), and southern areas of the former Soviet Union (26%). In contrast, only 0.1% of stools examined at a children's hospital in Calgary were positive for H nana. Most cases with associated neurologic symptoms have been reported from the former Soviet Union.
Morbidity/mortality
Morbidity is uncommon, only occurring when parasite burden is very high. Death has not been reported in association with this infection.
In a study of Bolivian schoolchildren, H nana infection was associated with an increased risk of anemia (odds ratio, 2.9; 95% confidence interval: 1.5-5.7; P=.002). [8]
Race-, sex-, and age-related demographics
No racial predisposition is known for hymenolepiasis.
No gender predilection is known for hymenolepiasis.
Disease is most prevalent in school aged children, up to 50% in children between 1-4 years of age. [7]
-
Life cycle of Hymenolepis nana. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
-
Life cycle of Hymenolepis dimunita. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).
-
H nana egg in an unstained wet mount. Courtesy of the Centers for Disease Control and Prevention (CDC at http://www.cdc.gov/dpdx/hymenolepiasis/index.html).






