Practice Essentials
Smoke inhalation is the leading cause of death due to fires. It produces injury through several mechanisms, including thermal injury to the upper airway, irritation or chemical injury to the airways from soot, asphyxiation, and toxicity from carbon monoxide (CO) and other gases such as cyanide (CN). See the image below.
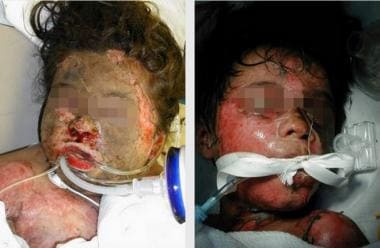 Smoke inhalation in pediatric victims. Note the many hallmarks of smoke inhalation complexed with burn injury (ie, facial burns, carbonaceous particles in the nasal cavity, periorbital edema, hair singeing). Early endotracheal tube placement is necessary to secure patency of the upper airways and adequate ventilation.
Smoke inhalation in pediatric victims. Note the many hallmarks of smoke inhalation complexed with burn injury (ie, facial burns, carbonaceous particles in the nasal cavity, periorbital edema, hair singeing). Early endotracheal tube placement is necessary to secure patency of the upper airways and adequate ventilation.
Signs and symptoms
Findings on physical examination may include the following:
-
Facial burns
-
Blistering or edema of the oropharynx
-
Hoarseness
-
Stridor
-
Upper airway mucosal lesions
-
Carbonaceous sputum
Symptoms of lower respiratory tract injury include the following:
-
Tachypnea
-
Dyspnea
-
Cough
-
Decreased breath sounds
-
Wheezing
-
Rales
-
Rhonchi
-
Retractions
Cyanosis may be present. However, cyanosis is an unreliable indicator of hypoxia because neither carbon monoxide nor CN cause cyanosis.
Findings in patients exposed to asphyxiants may include the following:
-
CNS depression, lethargy, and obtundation
-
Irritability, severe temporal headache, and generalized muscle weakness
-
Coma (nearly always from CO poisoning)
See Clinical Presentation for more detail.
Diagnosis
Studies may include the following:
-
Pulse oximetry and CO-oximetry
-
Arterial blood gases (ABGs)
-
Carboxyhemoglobin level
-
Lactate
-
CBC
-
Chest radiography (in patients with significant exposure or pulmonary symptoms)
-
ECG
-
Serial cardiac enzymes (in patients with chest pain)
-
Pulmonary function testing
-
Direct laryngoscopy and fiberoptic bronchoscopy
Carboxyhemoglobin levels in the blood and the corresponding clinical manifestations are as follows [1] :
-
0-10% - Usually no symptoms
-
10-20% - Mild headache, atypical dyspnea
-
20-30% - Throbbing headache, impaired concentration
-
30-40% - Severe headache, impaired thinking
-
40-50% - Confusion, lethargy, syncope
-
50-60% - Respiratory failure, seizures
-
>70% - Coma, death
Blood carboxyhemoglobin levels may underestimate the degree of CO intoxication because of oxygen administered to the patient before arrival to the hospital. The use of nomograms to extrapolate levels to the time of rescue has been shown to have prognostic value.
See Workup for more detail.
Management
When a patient presents with smoke inhalation, immediate assessment of the patient’s airway, breathing, and circulation should be done. [2] Provide IV access, cardiac monitoring, and supplemental oxygen in the setting of hypoxia. Some patients manifest bronchospasm and may benefit from the use of bronchodilators. When upper airway injury is suspected, elective intubation should be considered. Airway edema can progress over the next 24-48 hours and may make later intubation difficult if not impossible. Studies have shown that initial evaluation is not a good predictor of the airway obstruction that may ensue later secondary to rapidly progressing edema. [2]
Although controlled studies assessing the effects of steroids on various forms of chemical pneumonitis are disappointing, steroids have been suggested as having some value in exposure to the following [3] :
-
Oxides of nitrogen (NOx)
-
Zinc oxide (HC)
-
Red phosphorus (RP)
-
Sulfur trioxide (FS)
-
Titanium tetrachloride (FM)
-
Polytetrafluoroethylene (PTFE; Teflon)
Patients with smoke inhalation should be monitored for 4-6 hours in the ED. Those who are at low risk for injury and whose vital signs and physical examination findings remain normal can usually be discharged with close follow-up and instructions to return if symptoms develop. Patients with any of the following should be strongly considered for hospitalization:
-
History of closed-space exposure for longer than 10 minutes
-
Carbonaceous sputum production
-
Arterial PO2 less than 60 mm Hg
-
Metabolic acidosis
-
Carboxyhemoglobin levels above 15%
-
Arteriovenous oxygen difference (on 100% oxygen) greater than 100 mm Hg
-
Bronchospasm
-
Odynophagia
-
Central facial burns
Mechanical ventilation may be necessary in patients with declining lung function, oxygenation levels, and ventilation. It is given as follows:
-
Positive pressure ventilation with low tidal volumes (3-5 mL/kg)
-
Positive end-expiratory pressure (PEEP), with plateau pressures below 30 cm water
Neurologic abnormalities and a history of loss of consciousness are the primary clinical features used to define severe CO toxicity and are indications for hyperbaric oxygen (HBO) therapy. In addition, HBO use is indicated in patients with any of the following:
-
Base excess lower than -2 mmol/L
-
CO level greater than 25% (or >15% in pregnancy, as fetal hemoglobin binds CO more tightly)
-
Signs of cerebellar dysfunction
-
Cardiovascular dysfunction
-
Pulmonary edema
-
Extremes of age
See Treatment and Medication for more detail.
Background
Smoke inhalation injury was described as early as the first century CE, when Pliny reported the execution of prisoners by exposure to the smoke of greenwood fires. Smoke—the vaporous colloidal system formed when a material undergoes combustion or pyrolysis—comprises a collection of noxious gases, airborne solid particles, and airborne liquid particles. The distribution of those particles in the respiratory tract after inhalation is determined by their size and by the person’s breathing mechanics and tidal volume.
During fires, smoke inhalation victims are unable to efficiently breathe through the nasopharynx, thereby decreasing inspiratory air filtration and enabling a greater amount of particle distribution in the airway. This subsequently leads to nasopharyngeal irritation and severe lung injury.
Inhalation injury from smoke in fires may account for as many as 60-80% of fire-related deaths in the United States, many of which are preventable. [4, 5] Excellent care rendered at today's burn centers has greatly reduced the mortality from surface burns, [6] while the mortality from pulmonary injury has been increasing.
Many victims of fire accidents have both smoke inhalation and thermal injury. In fact, the co-presence of bronchopulmonary injury with cutaneous burns that exceed 30% of the total body surface area causes the mortality rate to increase more than 70%. [7] Other studies have shown that the incidence of inhalation injury increases with increasing burn size. [2]
Smoke inhalation may produce injury through several mechanisms. Heated air from a fire can cause significant thermal injury to the upper airway. Particulate matter produced during combustion (soot) can mechanically obstruct and irritate the airways, causing reflex bronchoconstriction. Noxious gases released from burning materials include carbon monoxide (CO) and hydrogen cyanide (CN).
Smoke may also contain aldehydes from combustion of furniture and cotton, and a variety of chemicals released by burning of rubber and plastics, including the following:
-
Chlorine gas
-
Ammonia
-
Various acids
-
Ketones
Smokes and obscurants long have been used by the military as a means of hiding troops, equipment, and certain areas from view of the opposing forces and from engagement by weapons with electro-optical control systems. Although smokes typically are not used as direct chemical agents, they may produce toxic injury to skin, eyes, and all parts of the respiratory tract. [8] Smokes are also produced inadvertently in industry by explosion, by mechanical generation, or as a by-product of a chemical interaction.
Smokes associated with the military, industry, or both, include the following:
-
Oxides of nitrogen (NOx)
-
Zinc oxide (HC)
-
Red phosphorus (RP)
-
Sulfur trioxide (FS)
-
Titanium tetrachloride (FM)
-
Oil fog (eg, Smoke Generator Fog 2 [SGF2])
-
Pyrolysis of polytetrafluoroethylene (PTFE; Teflon)
The chemical property of smoke combined with burn injury induces a complex pathophysiologic process that results in hypoxic insult, early airway edema, and bronchoconstriction. [9]
Diagnosis of inhalation injury is not always straightforward, owing to poorly sensitive screening tests and, in many cases, the delay in manifestation of clinically significant symptoms until 24-72 hours after injury. In treatment of smoke inhalation, the most immediate concern is reversing cellular asphyxia and carbon monoxide (CO) and CN toxicity (see Treatment).
Although children are less likely than adults to experience significant smoke inhalation, it remains a serious and life-threatening problem in the pediatric population. Management of a child with burns and a coexistent inhalation injury requires a cohesive team of pediatric intensive care physicians, nurses, and burn specialists.
Children with burns have traditionally been cared for in adult burn units, but the increased availability of physicians, nurses, and ancillary staff trained in the care of severely ill pediatric patients makes the pediatric intensive care unit a superior environment. Understanding that children are not merely small adults is critical to preventing therapeutic errors and disastrous iatrogenic complications.
Exposure to metal fumes and fluorocarbons—systemic toxins typically released during industrial fires—is rare in the pediatric population. Children are less likely to be affected by systemic toxins than by toxins from household products and products of smoke, including CO and CN poisoning.
Pathophysiology
The 3 primary mechanisms that lead to injury in smoke inhalation are thermal damage, asphyxiation, and pulmonary irritation. The combination of these mechanisms can explain the pathophysiologic responses that alter the airway microenvironment with parenchymal damage and predispose smoke inhalation victims to respiratory insufficiency.
Thermal damage
Thermal damage is usually limited to the oropharyngeal area, in part due to the poor conductivity of air. In addition, heat dissipation in the upper airways and laryngeal reflexes help protect the lower lung areas from direct thermal injury. Animal experiments have shown that 142°C inhaled air cools to 38°C by the time it reaches the carina. Steam, volatile gases, explosive gases, and the aspiration of hot liquids provide some exceptions, as moist air has a much greater heat-carrying capacity than dry air.
Asphyxiation
Tissue hypoxia can occur via several mechanisms. Combustion in a closed space can consume significant amounts of oxygen, decreasing the ambient concentration of oxygen to as low as 10-13%. For victims in that setting, the decrease in fraction of inspired oxygen (FIO2) leads to hypoxia, even if they have adequate circulation and oxygen-carrying capacity. If sufficiently severe, hypoxia can lead to multiorgan dysfunction, which substantially raises morbidity and mortality.
Carbon monoxide
Carbon monoxide (CO) is a colorless, odorless gas produced by the incomplete combustion of carbon-containing compounds, such as wood, coal, and gasoline. It is a major component of the smoke produced in open fires.
CO causes tissue hypoxia by decreasing the oxygen-carrying capacity of the blood. Hemoglobin binds CO with an affinity more than 200 times greater than the affinity for oxygen. Other mechanisms contribute, as well. [10] CO causes a left shift in the oxyhemoglobin saturation dissociation curve, which reduces the ability of hemoglobin to unload oxygen.
The heart is particularly affected because CO binds with the heme molecules in myoglobin, decreasing facilitated diffusion of oxygen into muscle. Interaction of CO with myocardial myoglobin results in decreased myocardial contractility.
A classic study demonstrated that dogs breathing 13% CO died within 1 hour after carboxyhemoglobin (CO-Hgb) levels reached 54% to 90%. However, exchange transfusion with blood containing 80% CO-Hgb to otherwise healthy dogs resulted in no toxic effects, despite resultant CO-Hgb levels of 57-64%. This further supports the notion that CO toxicity is not dependent on CO-Hgb formation or, in other words, solely upon a relative anemia. [11]
The literature suggests that hypoxic encephalopathy secondary to CO poisoning results from a reperfusion injury in which the products of lipid peroxidation and free radical formation contribute to morbidity and mortality. The therapeutic effect of hyperbaric oxygen therapy in these patients is attributed to improvement in mitochondrial oxidative metabolism, impairment of adherence of neutrophils to cerebral vasculature (decreases inflammation), and preservation of adenosine triphosphate activity.
Cyanide
CN gas can be produced by combustion of the following:
-
Plastics
-
Polyurethane
-
Wool
-
Silk
-
Nylon
-
Nitriles
-
Rubber
-
Paper products
The incomplete combustion of nitrogen-containing materials releases hydrogen CN (HCN), a colorless gas with a bitter almond odor that is detectable by 40% of the population. The burning of cotton generates 130 µg HCN/g; of paper, 1100 µg HCN/g; and of wool, 6300 µg HCN/g. [12] CN is 20 times more toxic than CO and can cause immediate respiratory arrest.
CN directly stimulates chemoreceptors of carotid and aortic bodies, leading to a brief period of hyperpnea. [12] CN is a small lipophilic molecule and a chemical asphyxiant that interferes with cellular metabolism by binding to the ferric ion on cytochrome a3, subsequently halting cellular respiration. Affected cells convert to anaerobic metabolism, and lactic acidosis ensues.
The organs most sensitive to cellular hypoxia are the central nervous system (CNS) and the heart. The CNS reacts to low concentrations of CN by promoting hyperventilation, thereby increasing exposure.
Consider CN toxicity in all patients with smoke inhalation who have CNS or cardiovascular findings. CN toxicity is difficult to confirm but is frequently concomitant with CO toxicity. Its presence can be inferred by the presence of lactic acidosis in the right clinical setting. Even mild degrees of CN poisoning can cause delayed neurological sequelae in survivors and permanent disability including the following:
-
Seizures
-
Various extrapyramidal syndromes
-
Dystonia
-
Postanoxic coma
Methemoglobinemia
Methemoglobinemia occurs in fire due to heat denaturation of hemoglobin, oxides produced in fire, and methemoglobin-forming materials such as nitrites. Methemoglobinemia is less common in smoke inhalation injury than CN and CO toxicity. The pathophysiologic consequences of methemoglobin formation are a decrease in the oxygen-carrying capacity of the blood and a shift of the oxyhemoglobin dissociation curve to the left, similar to carboxyhemoglobin.
Pulmonary irritation
Pulmonary injury from smoke inhalation is characterized by both hyperinflation and atelectasis. Debris from cellular necrosis, inflammatory exudate, and shed epithelium combine with carbonaceous material to narrow airways that are already compromised by edema. Reflex bronchoconstriction further exacerbates the obstruction.
Both inspiratory and expiratory resistance are increased, and the premature closure of small airways occurs, producing hyperinflation and air trapping. Surfactant production and activity are both impaired, leading to alveolar collapse and segmental atelectasis.
Low-pressure pulmonary edema plays an important role in the development of lung injury from smoke inhalation. Damage to the alveolar capillary membrane increases its permeability, and intravascular leakage into the pulmonary interstitium ensues. Eventually, increased lymphatic flow may be overwhelmed, resulting in alveolar edema. Alveoli fill with thick, bloody fluid. Loss of compliance, further atelectasis, and increasing edema can result in severe ventilation-perfusion mismatch and hypoxia.
Pulmonary injury may also occur as a direct result of hypoxia. The decrease in ambient oxygen tension that occurs during fires in closed spaces depends on the substances that are burned. Gasoline self-extinguishes when oxygen concentrations fall below 15%. Other substances may continue to undergo thermal decomposition, further decreasing ambient oxygen tension. Even small decrements in oxygen tension have a potentiating effect on inhaled asphyxiant gases, such as CO and HCN, resulting in severe lactic acidosis and a high fatality rate.
Inhalation of toxic products triggers a cascade of effects in the lower lung areas, such as the following [13] :
-
Activation of the body's inflammatory response system
-
Direct tissue injury
-
Mucus oversecretion
-
Acute bronchospasm or bronchorrhea
Activation of polymorphonuclear neutrophils (PMNs), resident alveolar macrophages, increased activity of systemic interleukin (IL)–1, IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) and neutrophilic infiltration are suggested to mediate the physiopathologic changes that subsequently lead to atelectasis and impaired mucociliary function. [7] In addition, humoral mediators such as prostanoids and leukotrienes produce reactive oxygen species and proteolytic enzymes, which contribute to the development of pulmonary edema and the formation of airway fibrin clots and hypoxemia.
Supporting the importance of the inflammatory response in tissue destruction, some studies have shown that administration of the cyclooxygenase inhibitor ibuprofen reduced the lung lymph flow in animals with smoke inhalation. [14, 15]
The direct injury is a consequence of the size of the particle, its solubility in water, and its acid-base status. Ammonia produces alkaline injury, while sulfur dioxide and chlorine gas cause acid injuries. Other chemicals act via different mechanisms; for instance, acrolein causes free radical formation and protein denaturation. [16]
The solubility of the substance in water determines the location of injury and timing of symptoms. Highly soluble substances such as acrolein, sulfur dioxide, ammonia, and hydrogen chloride cause injury to the upper airway and cause immediate symptoms. Substances with intermediate solubility, such as chlorine and isocyanates, cause both upper and lower respiratory tract injury with more delayed symptoms. Phosgene (a colorless gas with oxidant properties) and oxides of nitrogen have low water solubility and cause diffuse parenchymal injury, with symptoms sometimes delayed up to a day or more.
Days after the injury, the risk of bacterial infection increases markedly. Ciliary function is impaired, leading to accumulation of airway debris. Macrophages within the alveoli are destroyed, allowing bacteria to proliferate. Disruption of the epithelial barrier by ulcerations and necrosis further facilitates the development of pneumonia.
Smokes associated with the military or industry
Smokes and obscurants long have been used by the military as a means of hiding troops, equipment, and certain areas from view of the opposing forces and from engagement by weapons with electro-optical control systems. Although smokes typically are not used as direct chemical agents, they may produce toxic injury to skin, eyes, and all parts of the respiratory tract. [8] Smokes also are produced in industry by explosion, by mechanical generation, or as a by-product of a chemical interaction.
Smokes associated with the military, industry, or both, include the following:
-
Oxides of nitrogen (NOx)
-
Zinc oxide (HC)
-
Red phosphorus (RP)
-
Sulfur trioxide (FS)
-
Titanium tetrachloride (FM)
-
Oil fog (eg, SGF2)
-
Teflon particles
Oxides of nitrogen
NOx are components of photochemical smog, usually approximately 0.053 ppm. Nitrogen dioxide exists as a mixture of nitrogen dioxide, a reddish brown gas, and nitrogen tetroxide, a colorless gas. Other forms of nitrogen oxide include nitrous oxide, which is a common anesthetic or (when inhaled without oxygen) asphyxiant, and nitric oxide, which quickly decomposes to nitrogen dioxide in the presence of moisture.
Inhalation of nitric oxide causes the formation of methemoglobin. Inhalation of nitrogen dioxide results in the formation of nitrite, which leads to a fall in blood pressure, production of methemoglobin, and cellular hypoxia. Inhalation of high concentrations causes rapid death without the formation of pulmonary edema.
More severe exposures result in production of yellow frothy fluid in the nasal passages, mouth, and trachea and marked pulmonary edema, which may be fatal. The symptoms following the inhalation of NOx are mostly due to nitrogen dioxide.
Zinc oxide
Zinc oxide is a constituent of HC smoke, also termed "white smoke," which was developed by the French and US Chemical Warfare Service after World War I. A number of reports have been published on various lung abnormalities occurring in military personnel and firefighters exposed to smoke from HC smoke bombs during training drills.
HC smoke is created by combustion of a mixture of equal amounts of hexachloroethane, zinc oxide, and approximately 7% grained aluminum or aluminum powder. When ignited, these release zinc chloride, and the pyrotechnic mixture of zinc chloride and hexachloroethane rapidly absorbs moisture from the air to form a grayish white smoke.
Other chemicals also are released in the combustion process, such as chlorinated hydrocarbons (eg, phosgene and carbon tetrachloride), chlorine gas, CO, and several other compounds. These most likely contribute to the observed respiratory effects.
HC has a sweetish acrid odor, even at moderate concentrations. HC exposure can produce a gradual decrease in total lung capacity, vital capacity, and diffusion capacity of carbon monoxide (DLCO). It also is associated with the development of pulmonary edema, increased airway resistance, and decreased compliance. These changes were found to be reversible with cessation of episodic exposure. [17]
While upper respiratory tract damage can occur from HC, the mean diameter of the primary smoke particles is approximately 0.1 micrometers, allowing them to reach the alveoli. In a retrospective cohort study of 20 patients, Hsu et al reported significant impairment of pulmonary function within 3-21 days following acute exposure. [18] Follow up in 1-2 months showed significant improvement with mild-to-moderate exposures, whereas severe exposures led to interstitial fibrosis and continued functional limitation.
In a study by Conner et al performed with guinea pigs, exposure to ultrafine HC particles (0.05 µm) in increasing degrees was associated with a dose-response elevation in protein, neutrophils, and angiotensin-converting enzyme found in lavage fluid. [19] A direct relationship also was observed with alkaline phosphatase, acid phosphatase, and lactate dehydrogenase in lavage fluid. Centriacinar inflammation was seen histologically, indicating evidence of pulmonary damage.
An interesting study by Marrs et al involving mice, rats, and guinea pigs demonstrated a positive association of alveologenic carcinoma in a dose-response trend to HC smoke as well as a variety of inflammatory changes. [20] These researchers concluded that hexachloroethane and zinc, as well as carbon tetrachloride (which may be present in HC smoke), may be animal carcinogens in certain circumstances. This raises the suspicion of HC as a potential human carcinogen.
Hepatotoxicity has also been described in humans exposed to HC smokes in enclosed spaces during military training. [21] The toxic effects appear to be primarily due to the chlorinated compounds produced by combustion: tetrachloromethane, tetrachloroethylene, hexachlorobenzene, and carbon tetrachloride. This last compound is well known for its hepatotoxicity. Acute exposure causes elevated liver enzyme levels by day 1 or 2, with a peak around day 18-21. Liver function test results should normalize by 6 weeks.
Metal fume fever
Metal fume fever (MFF) is a well-documented acute disease induced by intense inhalation of metal oxides. MFF is primarily associated with the inhalation of zinc oxide fumes that are produced when zinc-oxide coated steel (galvanized) or zinc containing alloys (eg, brass) is exposed to high temperatures. Keyes found that 1 in 5 welders has experienced MFF by age 30 years. [22]
MFF is a self-limited syndrome characterized by fever, myalgias, headache, and nausea. Symptoms develop 4-12 hours after exposure and typically last several hours; severe cases generally resolve in 1-2 days. Observation is usually all that is necessary.
The exact pathology of MFF is not well understood but likely involves the deposition of fine metal particulates in the alveoli. A study by Kuschner et al on human volunteers showed that pulmonary cytokines such as tumor necrosis factor (TNF), interleukin 6 (IL-6), and interleukin 8 (IL-8) may play important initial roles in mediating metal fume fever. [23]
Red phosphorus
After World War II, RP smoke was developed in an attempt to avoid the toxicity associated with the manufacturing of white phosphorus. RP is 95% phosphorus in a 5% butyl rubber base and provides an adequate tank screen on the battlefield.
When RP is oxidized, it forms a mixture of phosphorous acids. When these acids are exposed to water vapor, they in turn form polyphosphoric acids, which may be responsible for the toxic injuries to the upper airways. Most of these injuries are mild irritations. No human deaths have been reported from exposure to either white phosphorus or RP smokes.
Most of the pathologic consequences associated with phosphorus are from elemental white phosphorus fumes or vapor. Contact with elemental phosphorus can cause burns to body surfaces.
A well-described condition termed phossy jaw is associated with longer-term occupational exposures to airborne phosphorus fumes. This disease is a degenerative condition affecting the entire oral cavity including soft tissue, teeth, and bones. Massive necrosis of teeth, bone, and soft tissue can lead to life-threatening infections. Treatment typically consists of soft tissue and bone debridement, abscess drainage, and reconstructive surgery.
White phosphorus and RP smokes may cause respiratory tract irritation after 2-15 minutes of exposure. This probably is caused by the polyphosphoric acids that react with moist mucosal membranes. Respiratory tract irritation has been observed at concentrations of 187 mg phosphorus pentoxide equivalents per cubic meter for 5 minutes or longer. Intense congestion, edema, and hemorrhages were observed in lung tissue following a 1-hour exposure at varying concentrations in studies using rats, mice, and goats.
Sulfur trioxide
The smoke-producing agent FS, also known as sulfuric oxide, chlorosulfonic acid, or sulfuric anhydride, is typically a colorless liquid, which can exist as ice, fiberlike crystals, or gas. When it is exposed to air, it rapidly takes up water and forms white fumes. The smoke consists of 50% sulfur trioxide and 50% chlorosulfonic acid.
FS usually is dispersed by spray atomization. The sulfur trioxide evaporates from spray particles, reacts with surrounding moisture, and forms sulfur acid. The sulfur acid condenses into droplets that produce a dense white cloud. FS is extremely corrosive, which led to its disuse in by the military. However, it has industrial use as an intermediate in the production of sulfuric acid, as well as other chemicals and explosives. [24]
Toxicity from FS is that of an acidic irritation to mucosal membranes and even skin. The corrosive effect of acid on mucosa and keratinized skin causes significant irritations and chemical burns.
Titanium tetrachloride
Titanium tetrachloride, known as FM, is a colorless-to–pale yellow liquid that has fumes with a strong odor. Upon contact with water, it rapidly forms hydrochloric acid and titanium compounds. It is used to make titanium metal, white pigment in paints, and other products. It breaks down rapidly in the environment.
FM readily hydrolyzes in the presence of water or moist air via an exothermic reaction that occurs in 2 stages. First, FM reacts to form a highly dispersed particulate smoke. This smoke reacts with more moisture in the air to form hydrolytic products of FM such as hydrochloric acid, titanium oxychlorides, and titanium dioxide. Generation of the smoke has been used as screens in military operations. The formation of hydrochloric acid makes it irritating and corrosive.
When FM liquid is exposed to the air, it produces white fumes. These white fumes can come into contact with skin, resulting in a mild epithelial irritation that usually subsides within 24 hours. When mixed with water, FM produces both heat and hydrochloric acid, which can work synergistically to produce deep thermal burns.
The same pathophysiologic effects that occur with FS smoke occur with FM smoke, since both generate corrosive and irritating acids.
Oil fog
SGF2 is another type of chemical smoke obscurant used in the military. SGF2 is generated by injecting a light petroleum-based lubricating oil onto a heated engine exhaust manifold, causing the oil to vaporize and eventually recondense in the atmosphere. Any industry that generates an oil mist also may produce similar exposures. Petroleum oil smokes are the least toxic smokes. They seldom produce ill effects even after prolonged or multiple exposures.
Concentrations of oil mists in industrial settings range widely (0.8-50 mg/m3), with most at 3 mg/m3. The particle sizes also vary more than 1-5 µm in median diameter. They typically have a high molecular weight and are saturated hydrocarbons derived from distilled petroleum. Exposures to such smoke are likely to last for many hours in a single day or repeatedly over consecutive days.
Animal studies have demonstrated that chronic exposure to oil fog had no effect on pulmonary function endpoints such as total lung capacity, vital capacity, residual volume, DLCO, compliance, and end-expiratory volume. One exception exists; male rats exposed at 1.5 mg/L had decreased end-expiratory volume. Bronchiolar lavage and histopathology showed changes consistent with a mild inflammatory edema (ie, increased protein content, total cells, PMNs, macrophages).
Teflon particles
Teflon is used widely, for a variety of purposes (eg, as lubricants, insulators, nonstick coatings), in a range of industrial, commercial, and military settings. Closed-space fires in such settings have prompted studies of the toxicity of exposure to the by-products created from incinerated organofluorines. Pyrolysis of Teflon produces a particulate smoke that, if inhaled, produces a constellation of symptoms termed polymer fume fever (PFF).
Pyrolysis of Teflon occurs at approximately 450°C. Among the particles produced by pyrolysis is perfluoroisobutylene (PFIB), which appears to be the main cause of toxicity in PFF. The ultrafine particles initiate a severe inflammatory response at low inhaled particle mass concentrations, which suggests an oxidative injury. PMNs may regulate the inflammatory process with cytokine and antioxidant expression.
PFIB particles have an extremely rapid toxic effect on pulmonary tissues. Evidence of microscopic perivascular edema is observed within 5 minutes. Less intense exposures are followed by a latent period during which normal physiologic compensatory measures to control developing pulmonary edema ensue. Once these mechanisms are overcome, the time frame of which depends on the degree of exposure, the clinical syndrome of PFF follows.
More intense exposures also may produce a chemical conjunctivitis. Hemorrhagic inflammation of the lungs also can occur.
Etiology
Most often, inhalation injury results from direct damage to exposed epithelial surfaces and causes conjunctivitis, corneal edema, rhinitis, pharyngitis, laryngitis, tracheitis, bronchitis, bronchiolitis, and alveolitis. Systemic absorption of toxins also occurs. Ascertaining if respiratory insufficiency is due to direct pulmonary injury or is the result of the extensive metabolic, hemodynamic, and subsequent infectious complications of surface burns is difficult.
Inhalants are classified as irritants, asphyxiants, or systemic toxins. Irritants cause extensive cell injury within the respiratory tract. Systemic toxins are absorbed through the respiratory tract and go on to damage other organ systems. Toxic gases are liberated during the combustion of various substances, as listed in the table below.
Table. Inhalants [1, 25] (Open Table in a new window)
Type |
Inhalant |
Source |
Injury/Mechanism |
Irritant gases |
Ammonia |
Fertilizer, refrigerant, manufacturing of dyes, plastics, nylon |
Upper airway epithelial damage |
Chlorine |
Bleaching agent, sewage and water disinfectant, cleansing products |
Lower airway epithelial damage |
|
Sulfur dioxide |
Combustion of coal, oil, cooking fuel, smelting |
Upper airway epithelial damage |
|
Nitrogen dioxide |
Combustion of diesel, welding, manufacturing of dyes, lacquers, wall paper |
Terminal airway epithelial damage |
|
Asphyxiants (mitochondrial toxins) |
Carbon monoxidea |
Combustion of weeds, coal, gas, heaters |
Competes for oxygen sites on hemoglobin, myoglobin, heme-containing intracellular proteins |
Hydrogen cyanide (CN)b |
Burning of polyurethane, nitrocellulose (silk, nylon, wool) |
Tissue asphyxiation by inhibiting intracellular cytochrome oxidase activity, inhibits ATP production, leads to cellular anoxia |
|
Hydrogen sulfidec |
Sewage treatment facility, volcanic gases, coal mines, natural hot springs |
Similar to CN, tissue asphyxiant by inhibition of cytochrome oxidase, leads to disruption of electron transport chain, results in anaerobic metabolism |
|
Systemic toxins |
Hydrocarbons |
Inhalant abuse (toluene, benzene, Freon); aerosols; glue; gasoline; nail polish remover; typewriter correction fluid; ingestion of petroleum solvents, kerosene, liquid polishes |
CNS narcosis, anesthetic stats, diffuse GI symptoms, peripheral neuropathy with weakness, coma, sudden death, chemical pneumonitis, CNS abnormalities, GI irritation, cardiomyopathy, renal toxicity |
Organophosphates |
Insecticides, nerve gases |
Blocks acetylcholinesterase; cholinergic crisis with increased acetylcholine |
|
Metal fumes |
Metal oxides of zinc, copper, magnesium, jewelry making |
Flulike symptoms, fever, myalgia, weakness |
|
a Major component of smoke. b Smells like almonds, component of smoke from fires. c Smells like rotten eggs. |
|||
Carbon monoxide
Individual cases of CO poisoning can occur under many circumstances (eg, furnace malfunction, motor vehicle exhaust exposure). Multiple cases may occur after large-scale disasters that result in widespread, long-term power outages (eg, major hurricanes), where the residential use of portable gas generators in poorly ventilated areas creates the ideal situation for CO poisoning. In a study evaluating unintentional CO poisoning of Florida state residents from 1999-2007, 89% of all CO poisoning-related deaths were non–fire related. [26]
Oxides of nitrogen
At ground level, NOx are produced during electric or arc welding, combustion of fuels, detonation of nitrate-based explosives, combination of nitrogen-containing products, and decomposition of organic matter. Recently filled farm silos have high nitrogen dioxide levels for approximately 10 days, peaking at 4000 ppm. Significant quantities of nitrogen dioxide also are found in diesel engine exhaust.
Any person in one of the following occupational tasks or environments is at risk for accidental exposure to NOx:
-
Unventilated farm silos
-
Welding in confined spaces
-
Detonating nitrogen-based explosives in enclosed spaces (eg, tanks, ships)
-
Handling nitric acid
-
Resurfacing ice arenas
-
Using anesthetic gases
-
Missile fuel oxidizer spills
Zinc oxide
Since this smoke can be distributed by grenades, candles, pots, artillery shells, and special air bombs, any military personnel engaged in the use or activity of these tools are at risk for HC exposure. Exposure to zinc oxide also is common among welders and those who are engaged in the smelting of zinc.
Red phosphorus
Phosphorus smokes are used in military formulations for smoke screens, incendiaries, smoke markers, colored flares, and tracer bullets. Workers at phosphorus loading plants also can be exposed to phosphorus smoke.
Sulfur trioxide
Exposure to FS is an occupational hazard in the chemical and metal plating industries. FS exposure also may occur in the production of detergents, soaps, fertilizers, or lead-acid batteries (car batteries), in printing and publishing, or in photography shops. With reduced use of FS by the military, exposure in that setting has become less common.
Titanium tetrachloride
Because FM smoke breaks down so rapidly in the environment, people who work with it in industry seem to be most at risk. Because titanium tetrachloride is extremely irritating and corrosive in both the liquid formulation and the smoke formulation, its use has diminished.
Oil fog
Military personnel can be exposed to fine-particle oil fog when it is used in training or in combat. Similar exposures may occur in industrial settings where oil mists are created, such as the following:
-
Metalworking
-
Automobile and textile industries
-
Pressrooms
-
Mining
-
Die and mold lubrication
Teflon particles
Exposure to these fumes is common in closed-space fires where Teflon is pyrolyzed. In addition, polymer fume fever has been observed in persons with occupational exposure to Teflon powder whose cigarettes became contaminated with Teflon. [27]
Epidemiology
According to the US Fire Administration, from 2008-2017, the United States had an annual average estimate of 1,344,100 fires, which resulted in 3,190 civilian deaths, 16,225 civilian injuries, and $14.7 billion in direct property loss each year. [28] From January 1, 2021 to October 15, 2021, the US news media reported 1,757 civilian home fire fatalities. [29]
Fire death rates in the United States and Canada are twice as high as in Western Europe and Japan. [30] Burns and fires are the third leading cause of nontransport-related accidental death in all age groups in the United States. They comprise the second leading cause of death in the home for all ages and the leading cause of death in the home for children and young adults. [30] More than half of all fatal residential fires started between the hours of 11 pm and 7 am. [30]
The incidence of smoke inhalation increases from less than 10% in patients with a mean total body surface area (TBSA) burn size of 5% to more than 80% in patients with a mean TBSA burn size of 85% or more. Smoke inhalation is present in one third of patients treated at burn centers. The magnitude of smoke inhalation is devastating, as the presence of an inhalation injury has a greater effect on mortality than either patient age or surface area burned.
Racial, sexual, and age-related differences in incidence
One study in New Jersey reports on the demographics of fire fatalities and notes that victims who perished did not parallel the ethnic census of the time. [5, 30] Whites accounted for 53% of fatalities and comprised 49% on the census. African Americans accounted for 38% of fatalities and 13% of the census.
The male-to-female ratio in fire fatalities is about 3:2.
The New Jersey study also showed that children and the elderly represented a disproportionate percentage of people injured by fire. [30] People younger than 11 years or older than 70 years constituted 22% of the population but accounted for 40% of all fire fatalities. These statistics closely match national figures.
Small children are especially vulnerable because they are less likely to escape a confined space and they also have a higher minute ventilation, which increases exposure to smoke and other toxins released during pyrolysis. In addition, their relatively smaller airways are more severely affected by airway edema and obstructing material.
Burns in children are 2.5 times more likely to occur by scalding rather than flame exposure. Hence, the percentage of children who experience respiratory symptoms after burns is less than that of adults, who are more often exposed to smoke-producing flames. About 50% of all burn deaths are related to inhalation injuries. Early hypoxemia is a contributor to over 50% of smoke inhalation deaths, with CO intoxication accounting for as much as 80% of the fatalities.
A comprehensive study in Dallas, Texas looked at all house fires from 1991-1997. [31] Many of the findings parallel those of the New Jersey study. Relative risk of injury was 1.8 for men, 1.4 for boys, 2.8 for blacks, and 2.6 for elderly persons. In addition, among the injured, the proportion of injuries that were fatal was higher in persons older than 65 years (53%) and in those younger than 10 years (67%) compared with those aged 10-64 years (30%).
The lowest income tracts had the highest rate of injury. The rate of injury in households with a median income below $20,000 per year was 8 times that of tracts with a median income greater than $80,000 per year. In fact, tracts with extremely low incomes, less than $10,000 per year, had rates of injury 20 times that of the above.
Houses built in the 1950s and 1960s were somewhat more likely to burn than houses built before this time. This may be a case of "selection of the fittest" houses, with those houses most prone to burn having already done so, leaving the most structurally sound ones still standing.
Fires caused by arson occurred predominately in census tracts with lower median incomes. Eighty percent of fires occurred in homes with median incomes of less than $40,000 per year.
Causes of the house fires were as follows:
-
Arson (25.5%)
-
Electrical wiring/equipment (16.6%)
-
Heating equipment (15.8%)
-
Cooking (11.4%)
-
Smoking (5.5%)
-
Children playing with fire (4.5%)
-
Unknown/other (20.6%)
The rate of fire-related injury in houses in Dallas without a functioning smoke detector was 8.7 times that of homes with functioning smoke detectors. Houses that are most likely to have fires were least likely to have functioning smoke detectors. As a result of this study, a program in Dallas now provides and installs smoke detectors in census tracts with the highest rates of injuries and deaths related to house fires. [31]
Prognosis
Most inhalation injuries are self-limited and resolve within 48-72 hours. The severity of direct pulmonary parenchymal injury depends on the extent of exposure and the type of inhaled toxins produced during combustion. Most patients do not manifest spirometry changes. Rare long-term sequelae include tracheal stenosis, bronchiectasis, interstitial fibrosis, and bronchiolitis obliterans.
The prognosis for mild-to-moderate exposures of toxic smokes is generally very good, with the usual outcome return to full recovery without sequelae. Metal fume fever is self-limited and usually resolves after a short period of observation. Exposure to white smoke (HC) in a military setting can lead to acute lung injury and in severe cases to ARDS. With more severe exposures, lungs may become severely damaged and develop chronic pulmonary fibrosis.
Children with acute pulmonary injury from toxic inhalations generally do well once supported through the initial period of inflammation and damage. Most of the pulmonary damage is self-limited and resolves within 2-3 days. The degree of recovery depends on the extent of the pulmonary parenchymal injury and subsequent hypoxic damage to the organs.
Complications
Complications may include the following:
-
Pulmonary edema (4-9%)
-
Pneumonia (3-23%)
-
Atelectasis (1-5%)
Bacterial pneumonia often complicates inhalation injury within 4-5 days of presentation. This additional cellular damage can cause significant mortality days to weeks after the initial injury.
Exacerbation of pre-existing respiratory disease (eg, asthma, chronic obstructive pulmonary disease) is a potential sequela. [32] A study of firefighters showed certain subgroups to have an exaggerated decline in postexposure forced expiratory volume in 1 second (FEV1) that was not predicted by age, smoking history, intensity of exposure, or the use of self-contained breathing apparatus. [33] Some of these patients, who had no family history of reactive airway disease, went on to need long-term beta-agonist therapy. Exposures to some smokes, such as isocyanates, can cause asthma in people with no history of the disease.
Airway hyperreactivity generally improves over several months following inhalation injury. However, some authors documented long-term respiratory symptoms such as cough, wheeze, and shortness of breath even after mild inhalation injury. The long-term effects of inhaled toxins on pulmonary function are not yet determined.
Carbon monoxide poisoning
Concurrent CO poisoning and inhalation of other products of combustion can cause hypoxemia, end-organ injury, and morbidity. CO intoxication is a particularly serious consequence of smoke inhalation and may account for as much as 80% of fatalities from inhalation injury.
Most patients who arrive alive to the emergency department make a full recovery. However, up to one third of patients with significant CO exposure develop a secondary syndrome of long-term neurologic or neuropsychiatric dysfunction within 1-3 weeks after exposure. Higher cortical functions seem most severely affected. Whether the use of hyperbaric oxygen (HBO) therapy alters the outcome of delayed-onset neurologic dysfunction from CO poisoning remains controversial.
Mortality
Severe pulmonary injury, edema, and the inability to oxygenate or ventilate can result in death. A study of the characteristics of survivors and casualties of fire fatalities found that the following specific risk factors seem to elevate the rate of mortality [34] :
-
Age: Elderly persons (>64 y) and young children (< 10 y) are the most likely to die as a result of a fire
-
Persons having a physical or cognitive disability have a higher mortality rate than matched controls, as do persons under the influence of alcohol or other drugs; for these vulnerable populations, if a nonvulnerable potential rescuer was present, the fatality rate dropped from 49% to 39%
-
The absence of a functioning smoke detector increases the risk of death in a fire by about 60%
The combination of cutaneous burn and smoke inhalation results in far higher mortality rates than occur with either injury in isolation. In patients with a burn and no associated smoke inhalation or respiratory failure, the mortality rate is less than 2%. In patients with smoke inhalation alone and no burn or respiratory failure, the mortality rate is 7%. For patients with both a burn and smoke inhalation, the mortality rate increases to 29%, suggesting that the burn wounds themselves put an additional stress on the compromised lung. [35]
In a study by Chou et al of 150 children with CO poisoning who were referred for hyperbaric oxygen therapy, none of the children with CO poisoning alone died, compared with 22.6% of those with the combination of CO poisoning and smoke inhalation. Among children who died, 60% had the following combination of risk factors [36] :
-
Smoke inhalation
-
Low body temperature on arrival in the emergency department
-
High initial carboxyhemoglobin level
-
Respiratory and cardiac arrest in the field
Patient Education
Clinicians should encourage the use of smoke and CO detectors in the home, which can decrease the risk of injury by permitting early escape. Regular checks to ensure that the detectors are functioning should be part of the household routine. In one study, the presence of a functioning smoke detector lowered the risk of death in a residential fire by over 60%. [31]
Programs aimed at educating young children about the dangers of playing with lighters and matches and programs teaching families how to safely escape from burning buildings should be used to further limit the number of children experiencing inhalation and burn injury. Anticipatory guidance during well child visits should include fire safety instructions.
For patient education resources, see the Lung and Airway Center, First Aid and Injuries Center, Procedures Center, and Poisoning Center, as well as Smoke Inhalation and Carbon Monoxide Poisoning.
-
Smoke inhalation in pediatric victims. Note the many hallmarks of smoke inhalation complexed with burn injury (ie, facial burns, carbonaceous particles in the nasal cavity, periorbital edema, hair singeing). Early endotracheal tube placement is necessary to secure patency of the upper airways and adequate ventilation.
Tables
What would you like to print?
- Overview
- Presentation
- DDx
- Workup
- Approach Considerations
- Pulse Oximetry and CO-oximetry
- Arterial Blood Gases
- Carboxyhemoglobin Level
- Lactate and Other Blood Studies
- Other Blood Studies
- CBC Count
- Cyanide Levels
- Radiography
- Computed Tomography
- Radionuclide Scintigraphy
- Pulmonary Function Testing
- Direct Laryngoscopy and Fiberoptic Bronchoscopy
- Show All
- Treatment
- Medication
- Questions & Answers
- Tables
- References









