Practice Essentials
Embryologic studies by Woodward et al at the turn of the 20th century shed light on the understanding of vascular congenital anomalies.These anomalies are encountered infrequently in everyday practice. They represent an heterogeneous group of isolated or multiple abnormalities that are sometimes associated with complex congenital syndromes. Most vascular anomalies affect the skin, though any organ system can be involved. Nearly all cutaneous congenital vascular anomalies are evident either at birth or within the first few weeks of life.
The presence of such lesions at birth and early childhood invokes concern and fear in parents and, in some cases, starts a protracted process of multiple visits to various specialists. Thus, it is mandatory to take the time and diagnose lesions appropriately early on and to ensure that a multidisciplinary team approach is used if the disease process warrants. The first step toward this goal is to obtain a careful history and a meticulous physical examination; these can distinguish between vascular tumors and malformations with a diagnostic accuracy exceeding 90%. [1]
A great deal of confusion surrounds the nomenclature and classification of congenital vascular abnormalities, and as a result, prompt proper diagnosis and appropriate treatment are often lacking. Alarmingly, as reported in one study, as many as one half of patients referred to specialty clinics for vascular anomalies were diagnosed and monitored incorrectly. [2]
Accordingly, as stated by Mulliken et al, [3] an appropriate start to any discussion of congenital vascular anomalies should include the distinction between vascular tumors (eg, hemangiomas) and vascular malformations (eg, capillary or lymphatic); the two entities are decidedly different (see Pathophysiology).
The topic of vascular anomalies is quite broad. This article serves as a general review covering the major tumors and malformations that the general surgeon or practitioner may encounter in practice. To minimize confusing nomenclature and to organize the discussion of the topic, this article adheres to the Mulliken-Glowacki scheme.
There is evidence in the literature to support the view that the management of these patients must be multidisciplinary (see Treatment). Vascular anomaly clinics should include pediatricians, pediatric vascular or oncologic surgeons, interventional radiologists, dermatologists, geneticists, plastic surgeons, and anesthesiologists, with occasional consultations for pediatric ophthalmologists and maxillofacial and orthopedic surgeons. All of these physicians should have experience in the management of pediatric patients with vascular tumors and malformations and be willing and able to work together for the patient's benefit, remaining conscious of the long-term impact of care on the patient's quality of life.
As mentioned, associated syndromes are not infrequent. A full discussion of all such syndromes is outside the scope of this chapter, but some of the predominant syndromes are briefly mentioned in later sections.
Pathophysiology
Whereas vascular malformations result from abnormal vessel embryogenesis in early fetal life, vascular tumors are endothelial neoplasms characterized by cellular proliferation and growth. Malformations may involve a single type of vessel (eg, capillary or lymphatic) or may be of a mixed variety. In clinical practice, malformations are designated by the predominant channel type and resultant rheologic character (ie, fast vs slow flow).
Vascular tumors encompass a broad range of lesions, including angiosarcomas and tufted angiomas, among others. However, the most common vascular tumor remains the hemangioma, a benign and self-limited lesion usually found in infants.;
Currently, various schemas are used to categorize vascular tumors and malformations, [4, 5] stemming from the original classification described by Mulliken and Glowacki, [3] which is based on the pathologic characteristics of the endothelium and the natural course of the lesion. A simplified outline of their original classification of vascular anomalies is as follows [6] :
Vascular tumors include the following:
-
Hemangioma - Congenital; infantile
-
Tufted angioma
-
Pyogenic granuloma
-
Hemangiopericythoma
-
Angiosarcoma
Vascular malformations include the following:
-
Capillary
-
Lymphatic
-
Venous
-
Combined - Arteriovenous malformation (AVM); arteriovenous fistula (AVF)
In 2014, the International Society for the Study of Vascular Anomalies (ISSVA) issued an updated official classification of vascular anomalies, [7] which was subsequently reviewed in 2018. [8] In this classification, vascular tumors are broadly divided into the following categories:
-
Benign
-
Locally aggressive or borderline
-
Malignant
Vascular malformations are broadly divided into the following categories:
-
Simple malformations
-
Combined malformations
-
Anomalies of major named vessels
-
Malformations associated with other anomalies
The ISSVA classification also specifies individual conditions within these categories.
Hemangiomas
Hemangiomas (see the image below) are true neoplasms. About 30% of them are apparent at birth, and 70% appear in the first few weeks of life; in contrast, vascular malformations are always present at birth, though they are not always readily apparent. The natural course of hemangiomas is one of early and rapid proliferation, followed by involution and spontaneous regression, with only one rare variant that may persist unchanged through an individual’s life. [9] They are more common in girls than in boys (3:1), and their proliferative phase is characterized by endothelial cell hyperplasia due to activation of vasoactive peptides from the mast cells and a mutlilayered basal membrane.
On immunohistochemistry (IHC) studies, hemangiomas stain positively for specific biologic markers, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), Lewis Y antigen, and glucose transporter (GLUT)-1, the latter being pathognomonic of infantile hemangioma (see the first image below). Conversely, vascular malformations are congenital dysmorphogenesis and thus are always present at birth, never regress, and often grow slowly over time (see the second image below). Malformations do not show sex predilection, have a normal vascular endothelium, and do not express specific immune biomarkers.
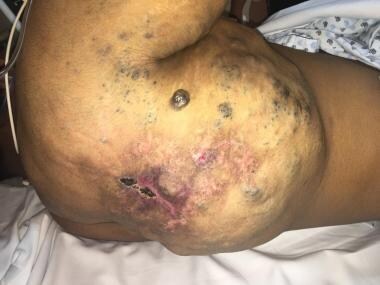 Clinical image of 9-year-old boy with axillary mixed malformation. Note increased volume, mostly due to lymphatic malformation, venous ectasia seen as cutaneous bluish varicose-looking venous malformation, and reddish port-wine stain due to capillary malformation.
Clinical image of 9-year-old boy with axillary mixed malformation. Note increased volume, mostly due to lymphatic malformation, venous ectasia seen as cutaneous bluish varicose-looking venous malformation, and reddish port-wine stain due to capillary malformation.
Some vascular malformations can expand and exhibit endothelial proliferation, usually after trauma, with endovascular or operative intervention, or during periods of hormonal change. Hence, as a rule, vascular lesions that persist into adolescence and adulthood are true vascular malformations and should not be referred to as hemangiomas.
Hemangiomas typically have the following three stages, classified on the basis of clinical assessment, microscopic morphology, and IHC markers [10] :
-
Proliferation phase (age 8 months to < 1 year)
-
Involuting phase (age 1-5 years)
-
Involuted phase (age >5 years)
Hemangiomas can be classified into two types, infantile and congenital. Infantile hemangiomas are generally cutaneous, arise in the cervicofacial region (60%), and may appear as focal, segmental, solitary or multiple. They tend to follow a predetermined course of proliferation and involution but exhibit wide variation in the rate, duration, and degree of growth and spontaneous regression. Unlike infantile hemangiomas, congenital hemangiomas present as fully-grown lesions at birth and do not undergo additional postnatal growth. [11]
On the basis of the natural history, the following three subtypes of congenital hemangioma have been identified [8] :
- Rapidly involuting congenital hemangioma (RICH) - 90% of involution has occurred by the age of 3 months of age, [12] and some lesions may be associated with thrombocytopenia or consumptive coagulopathy
- Partially involuting congenital hemangioma (PICH)
- Noninvoluting congenital hemangioma (NICH).
Most hemangiomas are small and pose only minor clinical problems before they involute and become clinically silent. However, about 20% pose significant problems and require treatment. [13] (See the images below.) This may result from aggressive growth, proximity to vital structures, or complications such as ulceration, bleeding, coagulopathy, or even high-output cardiac failure. [14] Finally, the disfiguring nature of certain lesions may prompt parents to seek intervention early rather than wait for the involution phase.
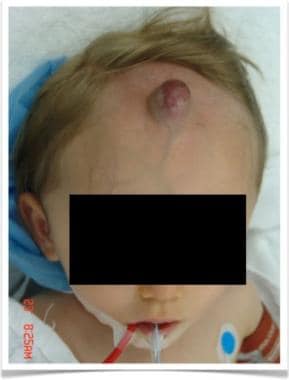 Preoperative image of 1-year-old boy with forehead hemangioma who required surgical resection because of previous episodes of massive bleeding after trauma. Initially, tissue expander was placed under scalp and progressively distended with water to achieve enough native tissue for defect coverage. Lesion was plastified before resection to reduce risk of operative bleeding.
Preoperative image of 1-year-old boy with forehead hemangioma who required surgical resection because of previous episodes of massive bleeding after trauma. Initially, tissue expander was placed under scalp and progressively distended with water to achieve enough native tissue for defect coverage. Lesion was plastified before resection to reduce risk of operative bleeding.
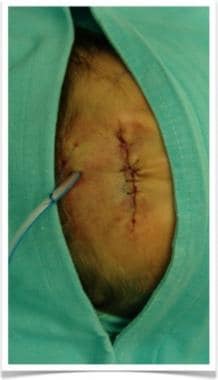 Postoperative image of previously mentioned patient with forehead hemangioma after surgical removal of hemangioma. Primary closure was achieved satisfactorily after extraction of tissue expander. Since dissection had been considerable, drain was left in place to avoid postoperative fluid collection, avoiding risk of palpebral and facial edema.
Postoperative image of previously mentioned patient with forehead hemangioma after surgical removal of hemangioma. Primary closure was achieved satisfactorily after extraction of tissue expander. Since dissection had been considerable, drain was left in place to avoid postoperative fluid collection, avoiding risk of palpebral and facial edema.
Vascular malformations
The pathophysiologic characteristics of vascular malformations are dictated by the type of channels involved (heme vs lymphatic) and the flow characteristics of the resultant lesion. Typically, capillary, venous, and lymphatic lesions tend to be slow-flow, whereas arterial lesions are fast-flow. Any combination of these elements is possible, resulting in an AVM, a capillary-lymphaticovenous malformation (CLVM), or a lymphaticovenous malformation (LVM). (See Presentation.) Venous malformations are most common.
CLVM with overgrowth of the lower extremity, mainly in girls, is referred to as Klippel-Trenaunay syndrome (KTS) (see the first and second images below). Because patients with KTS have deep venous system hypoplasia of the affected leg, they exhibit a pathognomonic dilatation of the superficial saphenous vein, called the marginal vein of Servelle (see the third and fourth images below).
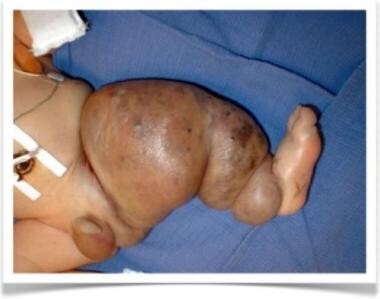 Clinical image of male newborn with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
Clinical image of male newborn with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
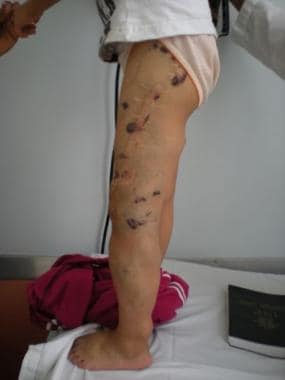 Clinical image of 7-year-old girl with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
Clinical image of 7-year-old girl with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
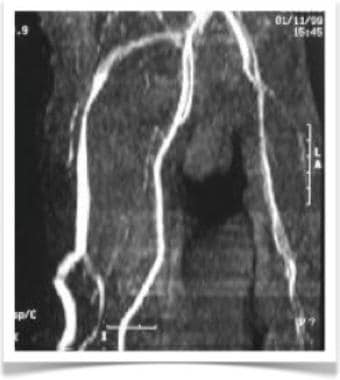 CT angiogram of patient with Klippel-Trenaunay syndrome demonstrating pathognomonic vein of Servelle. Hypoplasia of deep venous system of lower extremities causes dilatation of saphenous vein.
CT angiogram of patient with Klippel-Trenaunay syndrome demonstrating pathognomonic vein of Servelle. Hypoplasia of deep venous system of lower extremities causes dilatation of saphenous vein.
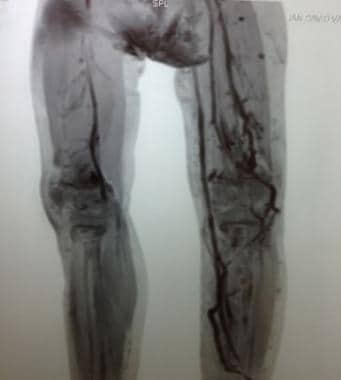 3D CT reconstruction of lower extremities in a patient with Klippel-Trenaunay syndrome. Hypoplasia of deep venous system of leg with subsequent tortuous dilatation of superficial venous system is evident.
3D CT reconstruction of lower extremities in a patient with Klippel-Trenaunay syndrome. Hypoplasia of deep venous system of leg with subsequent tortuous dilatation of superficial venous system is evident.
All vessel–type involvement (arteriovenous-capillary-lymphatic malformation) is referred to as Parks-Weber syndrome (see the images below).
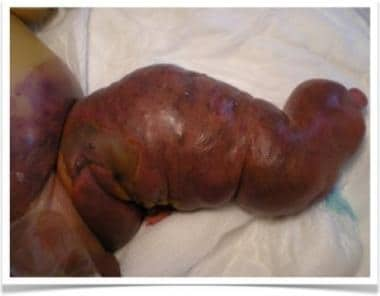 Newborn boy with Parks-Weber syndrome. Note diffuse left-lower-leg hypertrophy with larger overgrowth; lower abdominal and diffuse leg capillary malformation; tortuous venous dilatation of superficial system on left leg; and skin ischemia with necrosis and bleeding due to arteriovenous malformation.
Newborn boy with Parks-Weber syndrome. Note diffuse left-lower-leg hypertrophy with larger overgrowth; lower abdominal and diffuse leg capillary malformation; tortuous venous dilatation of superficial system on left leg; and skin ischemia with necrosis and bleeding due to arteriovenous malformation.
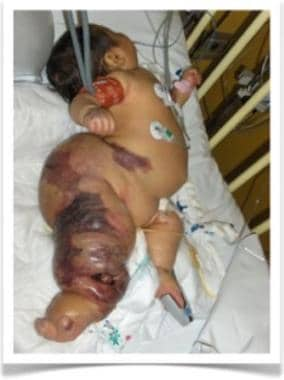 Newborn boy with Parks-Weber syndrome. Note severe overgrowth of right leg, with massive and diffuse mixed malformations. Port-wine stains represent capillary malformations, while arterial blood sequestration from arteriovenous malformation creates skin ischemia and necrosis.
Newborn boy with Parks-Weber syndrome. Note severe overgrowth of right leg, with massive and diffuse mixed malformations. Port-wine stains represent capillary malformations, while arterial blood sequestration from arteriovenous malformation creates skin ischemia and necrosis.
Etiology
The etiology of a particular vascular anomaly can vary greatly, depending on the nature of the lesion. In general, vascular tumors are endothelial neoplasms, the molecular biology of which remains poorly understood and characterized. There has been considerable interest in the mechanisms underlying the formation of hemangiomas, which are the most common of vascular tumors.
Although data from animal models remain lacking, research performed with human tissues has implicated numerous signal pathways that are altered during the various phases of hemangioma development. These include, among others, the following [15] :
-
Basic FGF (bFGF)
-
VEGF
-
Tissue inhibitor of metalloproteinases 1 (TIMP1)
-
Hypoxia-inducible factor (HIF)
Apart from these observational data, however, the mechanistic understanding of hemangioma development remains poor. Some authors have suggested a hereditary component to hemangiomas; however, the data are conflicting with respect to this notion. [16]
It has been suggested that vascular malformations arise from abnormalities in the process of normal vascular development. Specifically, perturbation of early angiogenesis and vasculogenesis may result in abnormal vascular channels, leading to the development of vascular malformations. [17] In contrast with vascular tumors, vascular malformations seem to have a strong hereditary component, with specific lesions observed in the setting of inherited syndromes.
Genetics
Alterations in several signaling molecules and pathways have been identified in specific types of malformations. For example, TIE2, [18] glomus cells, [19] and bFGF [6] have all been implicated in the formation of AVMs (see the image below). Investigators have localized chromosomal mutations underlying several combined vascular formation syndromes (eg, Klippel-Trenaunay-Weber syndrome and Proteus syndrome), further supporting a hereditary component to the development of AVMs. [20, 21]
The majority of mutations leading to vascular malformations are sporadic and post-zygotic, but other autosomal dominant germline mutations have been identified [22] . There are three signaling pathways in which the mutated genes are involved:
- PIK3CA/mammalian target of rapamycin (mTOR)
- RAS/mitogen-activated protein kinase (MAPK)
- G-protein coupled receptor signaling
The PIK3/AKT/mTOR pathway is an antiapoptotic pathway, in which mutations induce excessive and unregulated activation of the AKT pathway. [23] PIK3CA is one of the most relevant pathways by virtue of its relationship with the PROS (PIK3CA-related overgrowth spectrum) group lesions arising from somatic PIK3CA-activating mutations. It is also relevant to targeted pharmacotherapies, in particular to mTOR/PIK3CA/AKT pathway treatment with sirolimus (rapamycin), further discussed elsewhere (see Treatment). [22]
The penetrance and expressiveness of the genes involved in these pathways explain the different clinical phenotypes and underline the importance of high-sensitivity molecular diagnosis for targeted pharmacotherapy. [22]
Epidemiology
In general, vascular anomalies are rare, and limited data exist regarding their true worldwide incidence and prevalence. However, there is now a growing body of observations detailing the epidemiology and natural history of the more common subtypes of these anomalies (eg, hemangiomas and venous malformations).
Hemangiomas are the most common tumors of infancy and childhood, observed in 4-12% of infants during the first year of life. [13, 24] They are three to five times more common in females than in males. They are also more common in premature infants, with the risk increasing with lower birth weight. [25] The incidence among nonwhite populations remains unknown; however, hemangiomas in dark-skinned individuals are uncommon. [6]
Epidemiologic data regarding vascular malformations are also scarce. The overall incidence of congenital vascular malformations in the general population is estimated at 1.5%. Approximately two thirds of malformations are of venous predominance and are evenly distributed according to sex and race. [26]
According to the ISSVA and the German Interdisciplinary Society of Vascular Anomalies (DiGGefA), venous malformations are the most common representatives of vascular anomalies (70 %) (see the first image below), followed by lymphatic malformations (12 %) (see the second image below), AVMs (8 %), combined malformation syndromes (6 %) (see the third image below), and capillary malformations (4 %). [2]
In two separate case series, for example, patients with congenital vascular malformations were evaluated at Children's Hospital of Mexico City (1963-1983; 223 children) and the Walter Reed Army and National Naval Medical Centers (1984-1998; 169 children). Of the 392 patients, 257 (65.6%) had malformations of venous predominance. Prevalences of phlebectasia, aplasia or hypoplasia of venous trunks, aneurysms, and avalvulia were also recorded. [26]
Prognosis
Most hemangiomas have a self-limited course, leaving only a mild blemish or nearly imperceptible skin changes at the lesion site. However, the outcome of lesions that require intervention or operative management is heavily influenced by the nature and site of the lesion. For example, facial lesions may result in long-term cosmetic disfigurement.
Most vascular malformations represent progressive lesions, and long-term outcomes vary according to the nature, size, and location of a specific lesion. Lesions such as AVMs and small vascular malformations are more likely to be "cured" with surgical measures than lesions such as lymphatic malformations and mixed lesions are. With all of these lesions, however, long-term follow-up and vigilance are required to determine the efficacy of therapeutic intervention.
Although accurate diagnosis of these lesions may be complex, the promptness and precision with which it is achieved represents the basis for appropriate management. Accordingly, it is important to create awareness and spread knowledge of these conditions, [27] as well as to pay attention to their long-term implications (functional, emotional, and aesthetic) for a child's life.
-
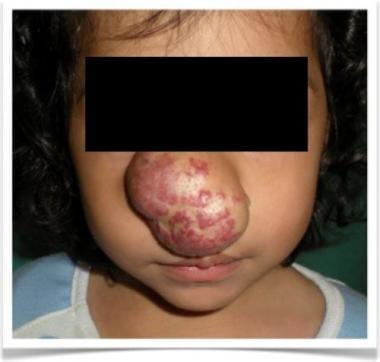
Disfiguring nasal hemangioma at proliferating phase on 2-year-old girl.
-
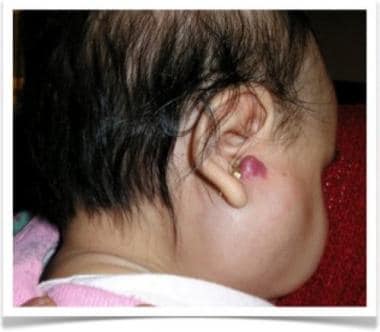
Deep and superficial hemangioma of parotid region in 4-month-old girl.
-
Clinical image of 9-year-old boy with axillary mixed malformation. Note increased volume, mostly due to lymphatic malformation, venous ectasia seen as cutaneous bluish varicose-looking venous malformation, and reddish port-wine stain due to capillary malformation.
-
Preoperative image of 2-year-old boy who underwent plastification of right-thoracic-wall arteriovenous malformation.
-
Surgical removal of AVM of same child described in previous image. Note how malformation can be resected en bloc without bleeding due to previous plastification.
-
Clinical image of 10-year-old boy with CLOVES syndrome (congenital lipomatous overgrowth syndrome with vascular anomalies, epidermal nevi, and scoliosis). Severe overgrowth on thorax, abdomen, and extremities, with multiple lipomas.
-
Clinical image of previously mentioned patient with CLOVES syndrome, from back. Scoliosis and severe left buttock enlargement are evident.
-
Closer clinical image of same patient with CLOVES syndrome, where lymphatic cutaneous infiltrate is seen as red confluent macules on right hemiabdomen.
-
Clinical image of 2-month-old boy with large hamartoma of right clavicular-axillary-thoracic region due to Proteus syndrome.
-
Surgical image of aforementioned patient with Proteus syndrome after removal of large axillary hamartoma.
-
Postoperative appearance of same patient with Proteus syndrome after soft-tissue reconstruction.
-
Patient who developed skin necrosis as a complication of sclerosis of a nontarget organ after endovascular injection of a sclerosing agent for a venous malformation.
-
Clinical image of 9-year-old girl presenting with cutaneous ulcer on right buttock as complication after endovascular sclerotherapy for venous malformation. Sclerosis of nontarget organ.
-
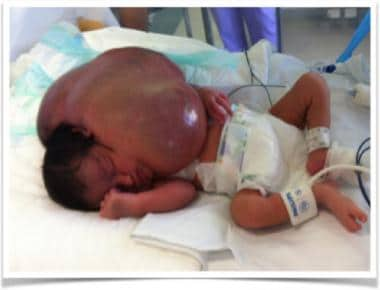
Newborn with massive macrocystic lymphatic malformation of left neck.
-
Preoperative image of 1-year-old boy with forehead hemangioma who required surgical resection because of previous episodes of massive bleeding after trauma. Initially, tissue expander was placed under scalp and progressively distended with water to achieve enough native tissue for defect coverage. Lesion was plastified before resection to reduce risk of operative bleeding.
-
Postoperative image of previously mentioned patient with forehead hemangioma after surgical removal of hemangioma. Primary closure was achieved satisfactorily after extraction of tissue expander. Since dissection had been considerable, drain was left in place to avoid postoperative fluid collection, avoiding risk of palpebral and facial edema.
-
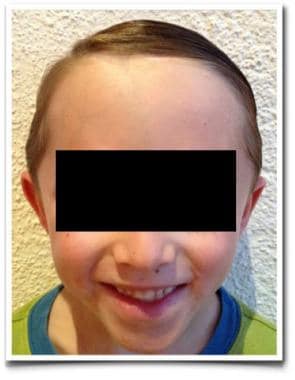
Clinical appearance of same patient with hemangioma at follow-up, aged 5 years.
-
Clinical image of 2-month-old boy with diffuse hemangiomatosis who presented with multiple cutaneous as well as hepatic hemangiomas.
-
Hemangioma of arm in aforementioned patient with diffuse hemangiomatosis.
-
Clinical image of right hemifacial mixed (venous and lymphatic) malformation in 7-year-old girl.
-
Clinical image of male newborn with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
-
Clinical image of 7-year-old girl with Klippel-Trenaunay syndrome. Note left leg hypertrophy, with evident cutaneous venous lesions.
-
Clinical image of 3-year-old boy who underwent percutaneous sclerotherapy with absolute alcohol for left-cheek venous malformation. Patient had very severe reaction to sclerosing agent and developed cutaneous lesion that scarred and retracted over time.
-
Clinical image of 14-year-old girl with Maffucci syndrome, comprising diffuse venous malformations and enchondromas. Venous malformations are readily visible on lips.
-
Clinical image of aforementioned patient with Maffucci syndrome demonstrating multiple enchondromas of wrist and fingers of left hand, combined with vascular malformations on distal phalanx of fifth finger.
-
Postoperative image of same patient with Maffucci syndrome after surgical resection of three venous malformations of lips.
-
Clinical picture of same patient with Maffucci syndrome with diffuse venous malformations on left foot.
-
CT angiogram of patient with Klippel-Trenaunay syndrome demonstrating pathognomonic vein of Servelle. Hypoplasia of deep venous system of lower extremities causes dilatation of saphenous vein.
-
3D CT reconstruction of lower extremities in a patient with Klippel-Trenaunay syndrome. Hypoplasia of deep venous system of leg with subsequent tortuous dilatation of superficial venous system is evident.
-
Surgical photograph during resection of axillary macrocystic lymphatic malformation that did not respond to sclerotherapy. Topographic anatomy of lesion required simultaneous two-sided (cervical and axillary) approach. Extreme care during axillary neurovascular structures is paramount for adequate postoperative functional outcome.
-
Postoperative image of aforementioned patient with axillary macrocystic lymphatic malformation.
-
Coronal CT image of abdomen in 2-year-old patient with intestinal macrocystic lymphatic malformation. Surgical treatment was chosen on basis of difficult percutaneous image-guided approach and high risk of complications (intestinal ischemia/perforation).
-
Surgical specimen of previously mentioned patient with intestinal macrocystic lymphatic malformation that required intestinal resection and primary anastomosis.
-
Clinical image of 3-year-old girl with large macrocystic lymphatic malformation on left neck.
-
Clinical image of newborn with large macrocystic lymphatic malformation and acute inflammation after upper respiratory tract infection.
-
Clinical image of 2-day-old male with massive mixed lymphatic malformation on left neck. Surgical treatment was chosen because of risk of airway compromise.
-
Sagittal T2-weighted MRI image of previously mentioned 3-year-old girl with large macrocystic lymphatic malformation on left neck.
-
Hemihypertrophy of right lower extremity on 2-month-old boy.
-
Severe mixed vascular malformation of right leg on 5-day-old boy, with massive overgrowth, port-wine stains, and diffuse venolymphatic malformation.
-
Newborn boy with Parks-Weber syndrome. Note diffuse left-lower-leg hypertrophy with larger overgrowth; lower abdominal and diffuse leg capillary malformation; tortuous venous dilatation of superficial system on left leg; and skin ischemia with necrosis and bleeding due to arteriovenous malformation.
-
Newborn boy with Parks-Weber syndrome. Note severe overgrowth of right leg, with massive and diffuse mixed malformations. Port-wine stains represent capillary malformations, while arterial blood sequestration from arteriovenous malformation creates skin ischemia and necrosis.
-
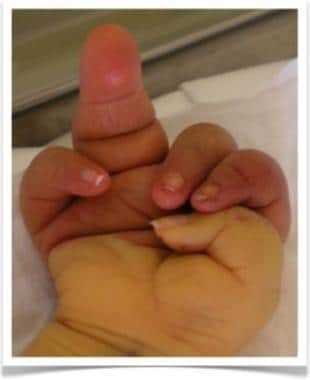
Clinical picture of right hand of 5-day-old boy with Parks-Weber syndrome. Note hypertrophy of fourth finger and port-wine stain (capillary malformation) on hand distal to crease.
-
Clinical picture of 5-year-old boy with arteriovenous vascular malformation of tongue. He underwent attempted surgical correction at local hospital, where surgeon attemtped to ligate primary inflow artery but instead ligated venous drainage vessel, provoking sudden and intense outflow obstruction with rapid lesion enlargement, tongue protrusion, and mucosal ischemia.
-
Preoperative image of aforementioned patient with arteriovenous vascular malformation of tongue, who initially received tracheostomy for airway control and feeding gastrostomy. Note severe tongue swelling and enlargement and poor mucosal perfusion with ischemia and necrosis.
-
Selective lingual artery angiography of aforementioned patient with arteriovenous vascular malformation of tongue demonstrates large, diffuse lingual arteriovenous malformation occupying entire tongue. At hemodynamics suite, with patient under general anesthesia, catheter was introduced through femoral artery and into external carotid artery, and micro-catheter was then passed through former into lingual artery. Under fluoroscopy with image substraction and contrast roadmapping, supraselective lingual artery branches were embolized.
-
Preoperative postembolization radiologic image of aforementioned patient with arteriovenous vascular malformation of tongue demonstrates important reduction of blood flow to malformation.
-
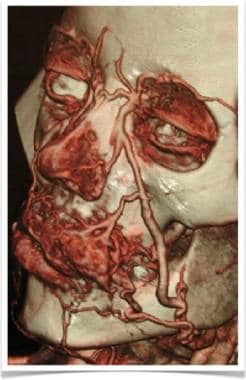
3D color reconstruction CT angiography depicts upper-lip venous malformation.
-
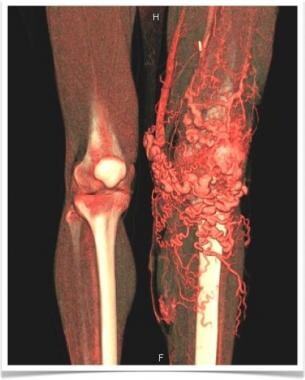
3D color reconstruction CT angiography depicts left-lower-extremity arteriovenous malformation.
-
Operative image of 6-year-old boy during postplastification glossectomy for lingual venous malformation.
-
Postoperative image after partial anterior glossectomy on previously mentioned 6-year-old boy.
-
Preoperative image of previously mentioned 6-year-old boy with marking of anterior partial glossectomy after plastification for treatment of lingual venous malformation.
-
Postoperative image after anterior two thirds glossectomy after embolization of lingual arteriovenous malformation.
-
Surgical specimen from anterior partial glossectomy after plastification for lingual venous malformation.